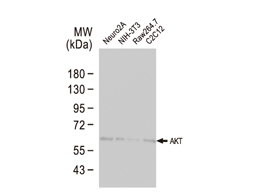
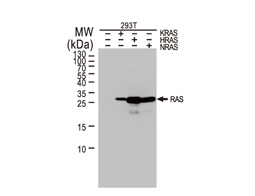
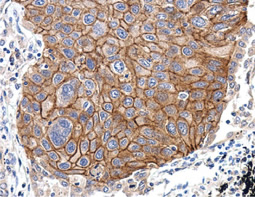
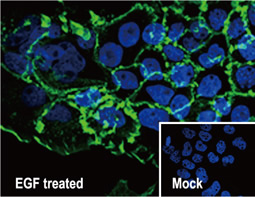
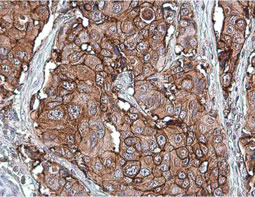
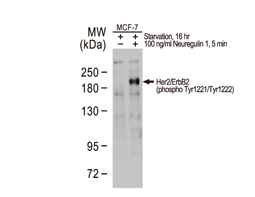
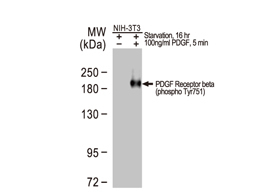
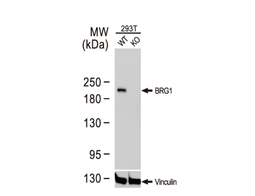
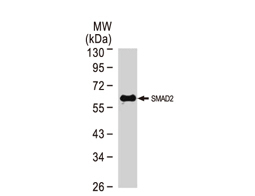
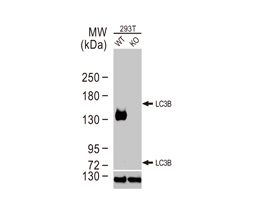
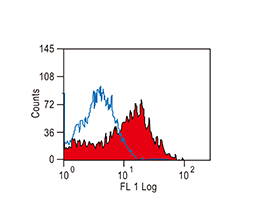
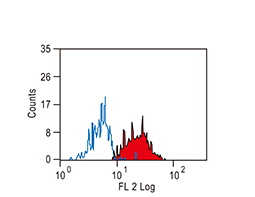
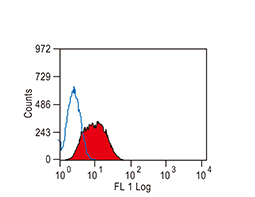
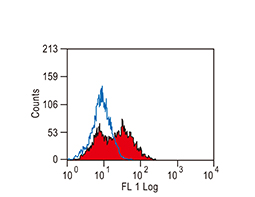
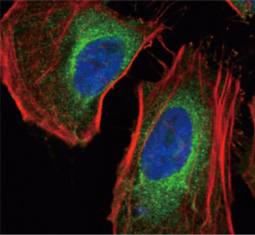
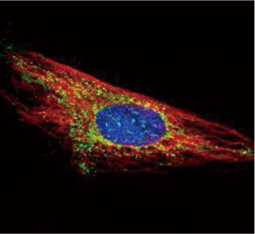
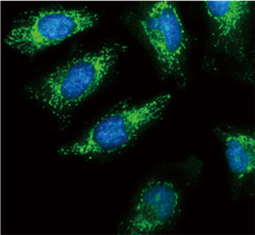
The products of metazoan cell proto-oncogenes are generally key constituents of the cell signaling and gene expression machinery that regulate cell growth and proliferation. When mutated, amplified, or otherwise expressed in a dysregulated manner, these genes can become cellular oncogenes and their oncoprotein products drive tumorigenic cell growth. Examples of proto-oncogenes include the RAS genes, ERBB2, MYC, and ABL1. Certain viruses, or oncoviruses, have the capability to cause cancers through the action of viral genome-encoded oncoproteins that target metazoan cell factors or pathways.
Cancer
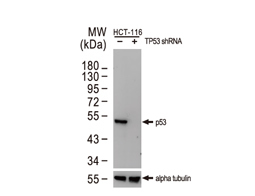
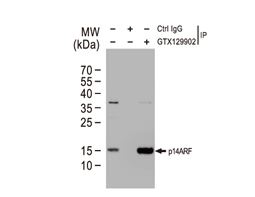
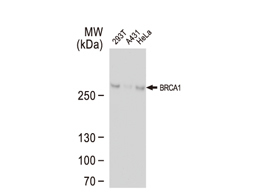
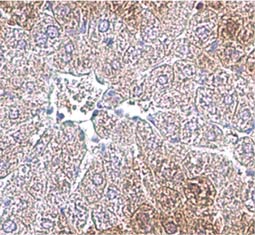
Tumor suppressor gene products, or tumor suppressor proteins, act as barriers to block cells from proceeding down the path to cancer. In contrast to the protein products of proto-oncogenes, some tumor suppressor proteins act to restrain abnormal cell proliferation. Others promote cell death pathways or function in DNA repair. While mutated proto-oncogenes behave in a dominant fashion, tumor suppressor genes are generally recessive, thus requiring that both be inactivated or otherwise compromised in order for cancers to occur. Well-described examples of tumor suppressor proteins include RB, p53, APC and CDKN2A/p14 ARF.
Highlighted Products
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
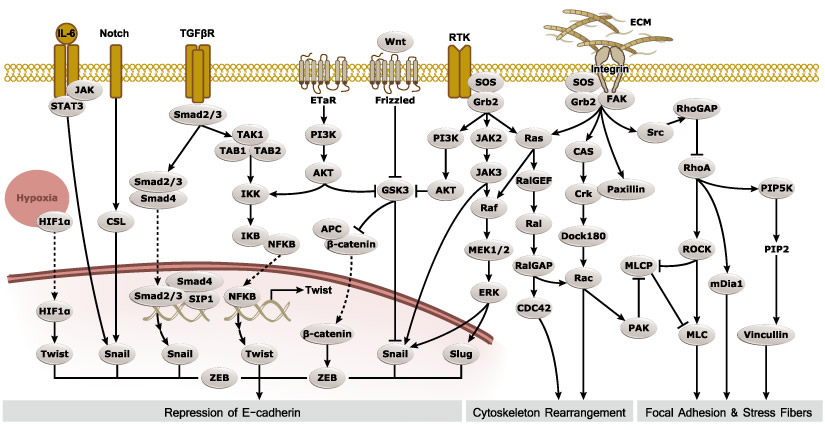

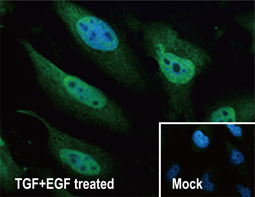
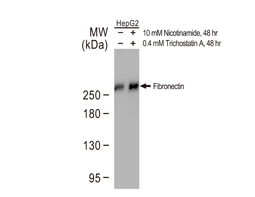
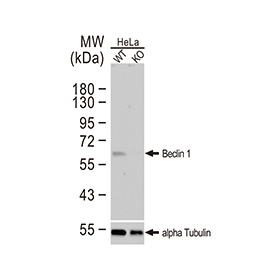
Epithelial-mesenchymal transition (EMT) is a process whereby largely adherent and polarized epithelial cells acquire the phenotype of more mobile mesenchymal cells. EMT not only facilitates morphogenesis during embryonic development but also promotes invasion and metastasis in tumors. Pathological EMT is associated with E-cadherin repression, which has been shown to contribute to tumor progression. Several oncogenic pathways (e.g., TGF-beta, Wnt/beta-catenin, integrins, and Notch) have been reported to induce EMT via cytoskeleton rearrangement and activation of E-cadherin repressors, including Snail, Slug, SIP1, ZEB1, and TCF3.
EMT-inducing Transcription Factors
|
|
|||||
Epithelial Markers
|
|
|||||
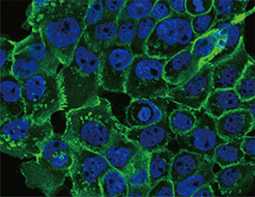

E-Cadherin antibody
|
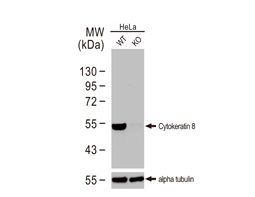

Cytokeratin 8 antibody
|
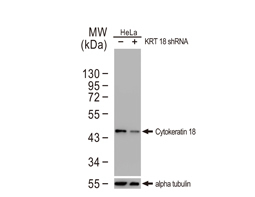

Cytokeratin 18 antibody
|
Mesenchymal Markers
|
|
|||||
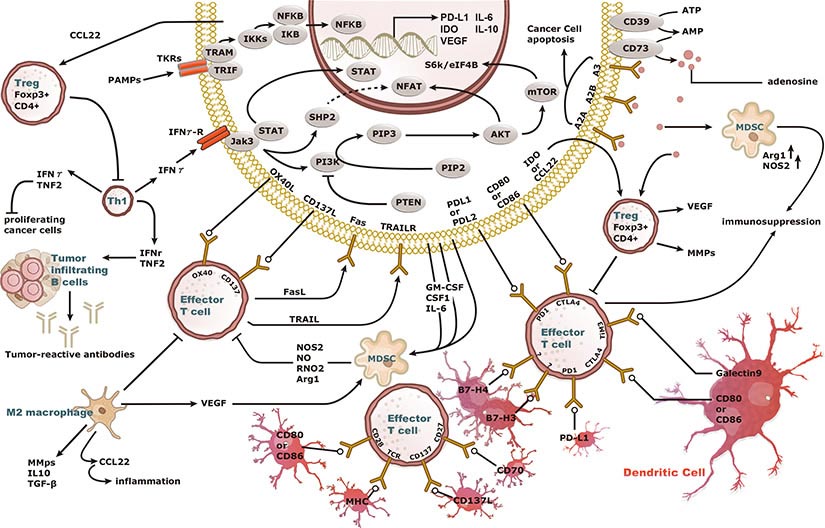

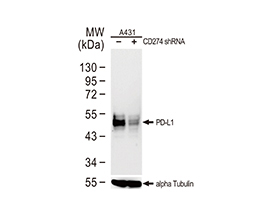
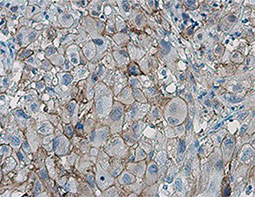
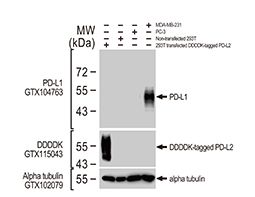
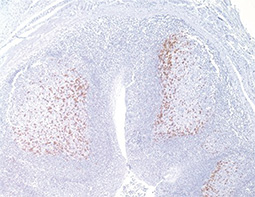
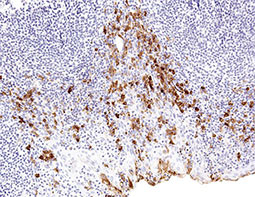
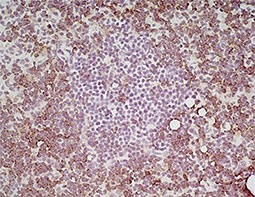
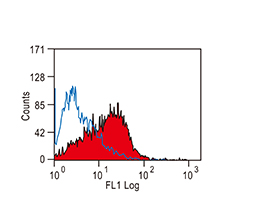
Increasing knowledge of how cancer cells escape immune destruction has significantly driven the evolution of cancer immunotherapy. In particular, studies describing how different immune checkpoints, including the PD-1 and CTLA-4 pathways, function to attenuate T cell antitumor activity have revealed novel opportunities for therapeutic intervention. Researchers have made promising strides in inhibiting these checkpoints to unleash the T cells’ tumor-killing potential. Given that both radiotherapy and chemotherapy can also mobilize antitumor T cells, there is great excitement that checkpoint inhibition and other immunomodulatory approaches can be combined with conventional modalities to maximize clinical outcomes for a number of malignancies.
GeneTex Proudly Provides an Outstanding PD-L1 Antibody for Your Research
• Used at 1:1000 for IHC
• Knockout/knockdown validation
• Highly specific and sensitive
• Citation support
| PD-L1 antibody (GTX104763) | |||||
|
|
|||||
| Highlighted Products | |||||
|
|
|||||
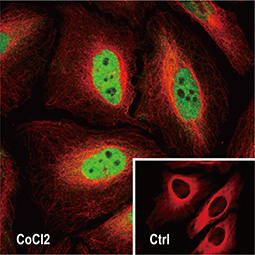
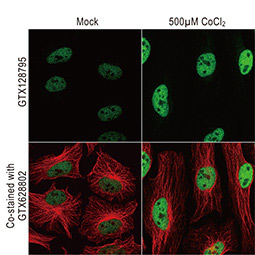
The 2019 Nobel Prize in Physiology or Medicine was awarded to William G. Kaelin Jr., Sir Peter J. Ratcliffe, and Gregg L. Semenza in recognition of their discovery of cellular mechanisms to sense and adapt to different oxygen concentrations, establishing a basis for how oxygen levels affect physiological function. Their work contributed new understanding of normal processes involving metabolism, embryonic development, and the immune response, among others. In addition, novel perspectives on pathological states including anemia, ischemia, infection, and cancer were derived from their research.
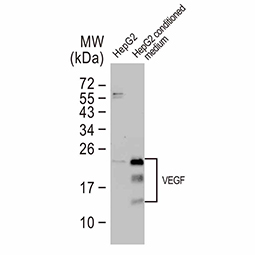
Dr. Semenza identified hypoxia-inducible factor (HIF-1α and ARNT, also known as HIF-1β), the primary transcription factor complex that drives expression of hypoxia-regulated proteins such as vascular endothelial growth factor (VEGF), erythropoietin (EPO), and carbonic anhydrase IX (CAIX). Drs. Kaelin and Ratcliffe described the proteasomal degradation of HIF-1α during normoxia through its interaction with the von Hippel-Lindau tumor suppressor protein (VHL), and how this interaction is enabled by prolyl hydroxylation (Fig. 1).
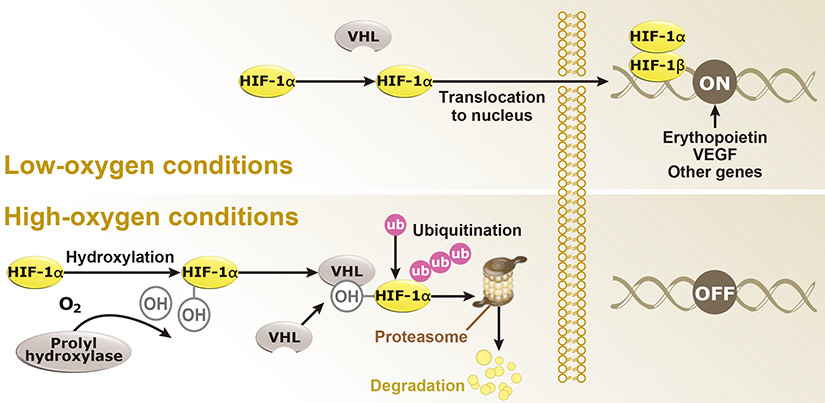

Fig. 1. Hypoxia regulation
Under hypoxic conditions (top), HIF-1α remains intact and enters the nucleus to interact with HIF-1β to effect gene expression that promotes hypoxia adaptability. In contrast, with normoxia (bottom), HIF-1α is hydroxylated by prolyl hydroxylases with subsequent binding to VHL, ubiquitination (Ub), and proteasomal degradation.
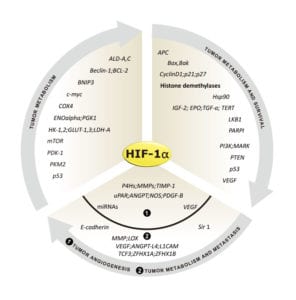
Genes regulated by HIF-1α (Fig. 2) are important for tumor metabolism, proliferation, survival, angiogenesis, and metastasis. Therefore, inhibition of HIF-1α and its related proteins has become a promising direction for anticancer therapy research.
Fig. 2. Genes that are regulated by HIF-1α
As oxygen supply in a tumor is frequently suboptimal, cancer cells often increase the expression of HIF-1α to stimulate gene expression that allows them to survive, proliferate, invade surrounding tissue, and metastasize to distant sites. In addition, the hypoxic tumor milieu can make cancer cells less sensitive to chemotherapy and radiotherapy.
Since 2012, GeneTex has been dedicated to the development of antibody reagents that facilitate hypoxia research. These products are rigorously evaluated for specificity and performance through our enhanced validation protocols, and are supported by multiple citations.
Highlighted Products
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()

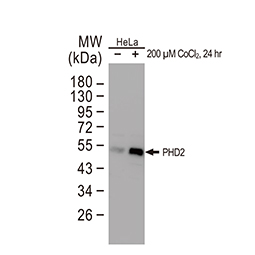
Von Hippel Lindau antibody
|

PHD1 antibody
|
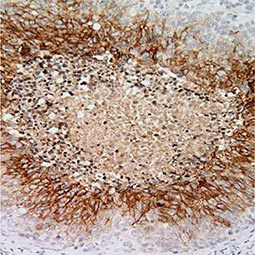

Carbonic Anhydrase IX antibody [GT12]
|
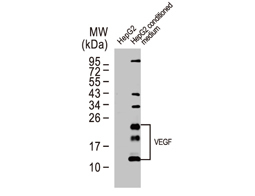
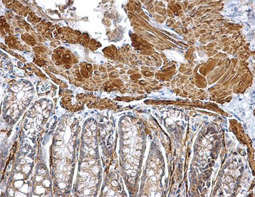
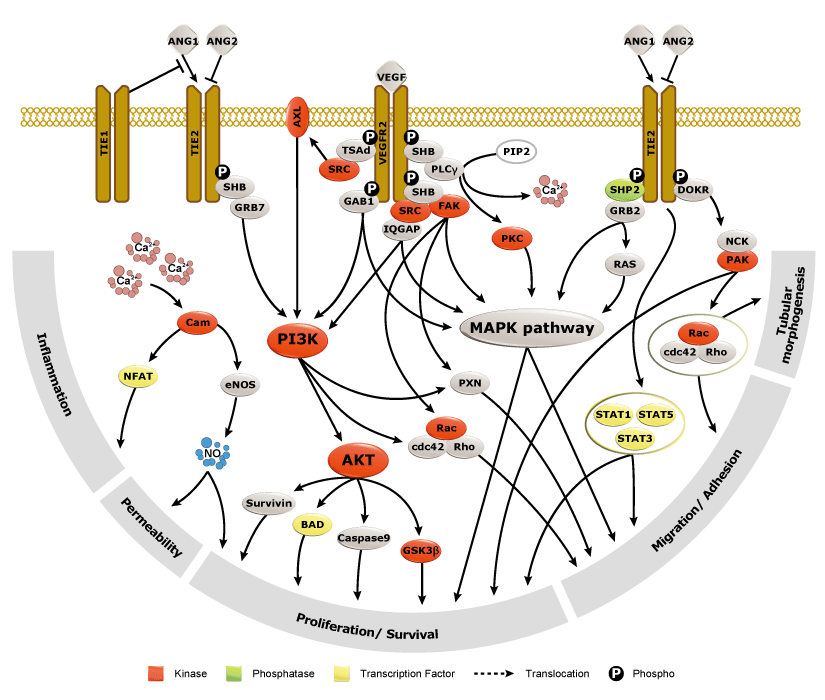
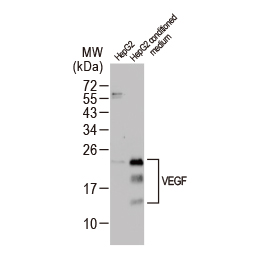
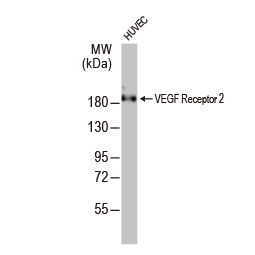
Angiogenesis entails the generation of new blood vessels from pre-existing vasculature. It is an intricate process tightly regulated by input from pro- and anti-angiogenic factors. In the normal adult, the balance between these inducers and inhibitors maintains a quiescent state that nevertheless is capable of rapid changes in response to micro-environmental disruptions secondary to wounds, hypoxia, or inflammation. Perturbations of this balance in various chronic pathological conditions and tumors can lead to inappropriate angiogenesis or neovascularization. The mechanistic features underlying angiogenesis are complex, but the clinical importance of being able to modulate this process means that this will remain a compelling and dynamic field of research.
GeneTex is proud to offer an extensive line of research antibodies to support the study of angiogenesis. Please see the highlighted products below or click the button to see more product information.
Highlighted Products
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
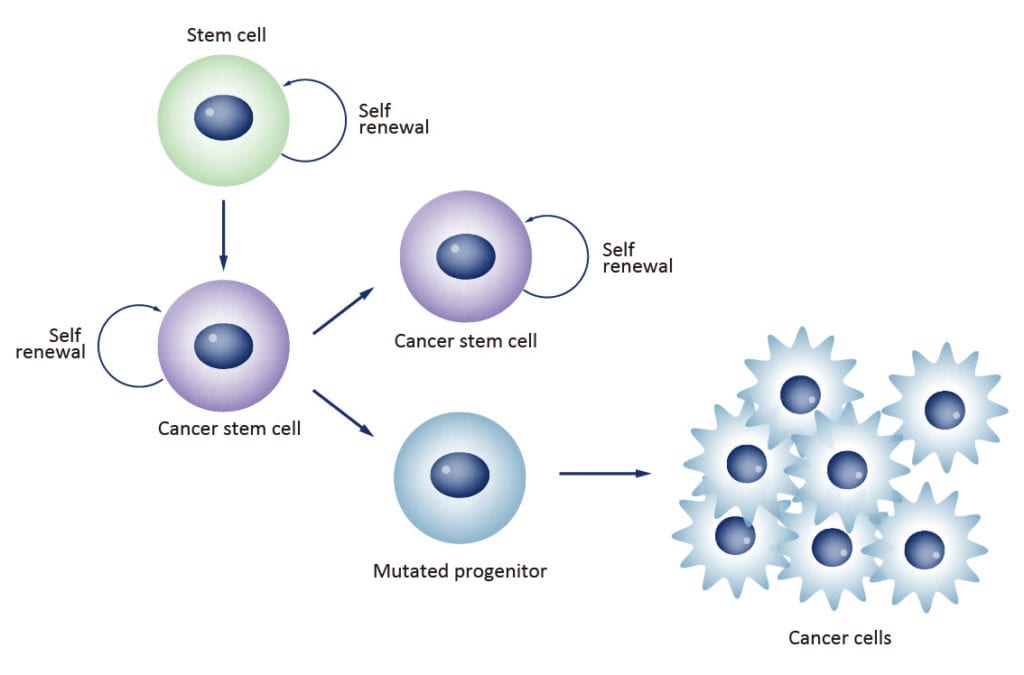

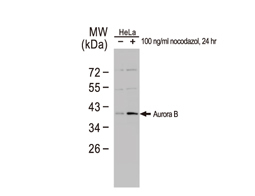
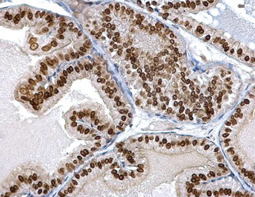
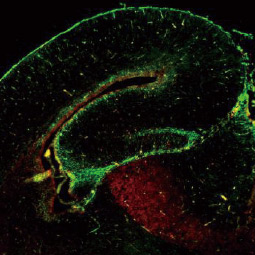
Cancer stem cells (CSCs) are increasingly being recognized as the key determinative cells of malignancies. CSCs are described as relatively quiescent cells capable of self-renewal and the generation of more differentiated daughter cells that constitute the bulk of the tumor mass. While there is still debate as to the existence of a single subset of CSCs versus a collection of CSC variants in some tumors, a growing body of literature links CSCs to all steps of tumorigenesis, from initiation to metastasis. Even more ominously, however, evidence suggests that these cells are responsible for therapeutic resistance and relapse, with their persistence often meaning a poor clinical prognosis. For these reasons, CSC biology has become a vigorously studied area of cancer research.
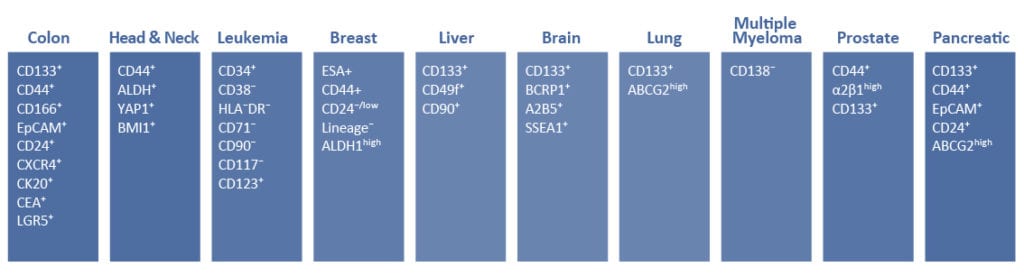

Highlighted Products
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()

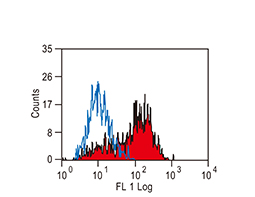
SOX2 antibody [GT1876],
|
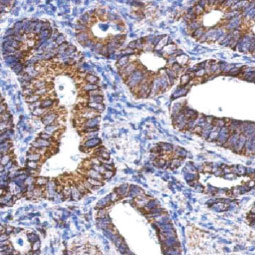

ALDH1A1 antibody
|


CD44 antibody [GT981]
|
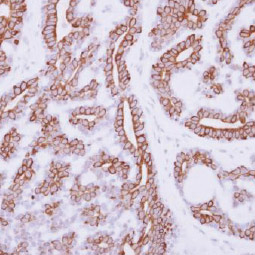

IL3 Receptor alpha antibody [N2C2], Internal
|
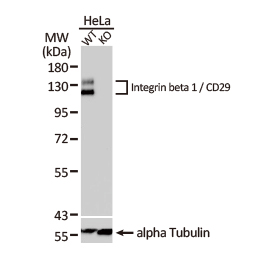

Integrin beta 1 / CD29 antibody
|
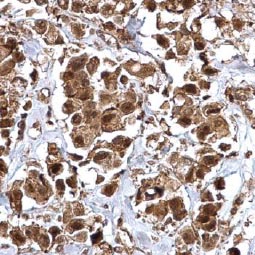

Bmi1 antibody
|
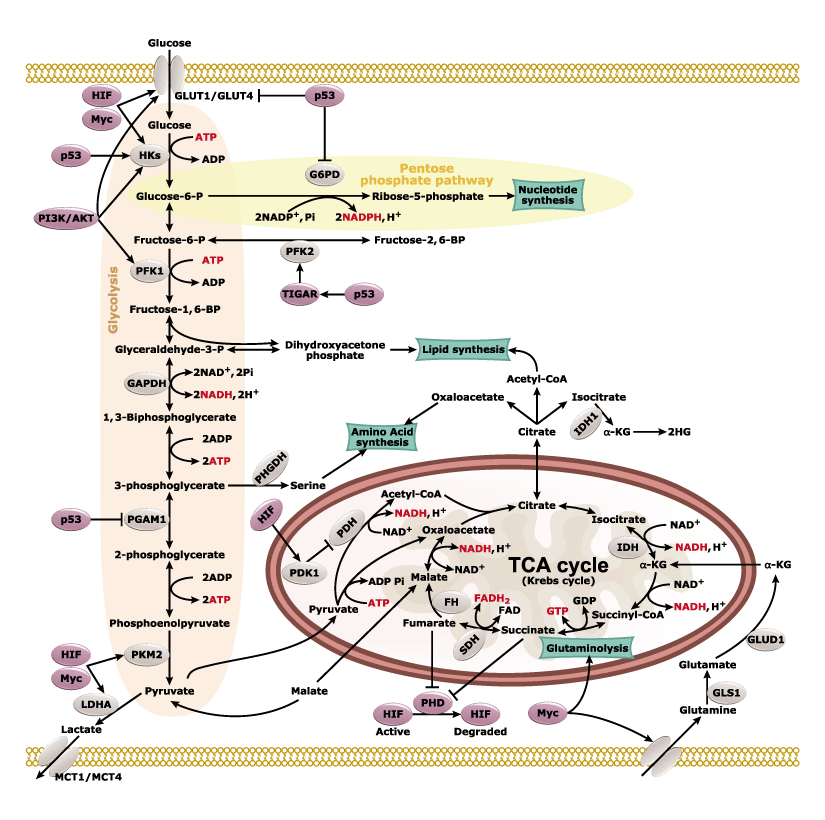
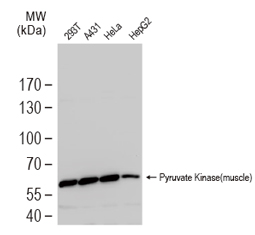
Nearly a century ago, Otto Warburg found that cancer cells can shift their energy production to aerobic glycolysis instead of the more efficient oxidative phosphorylation. An emerging hallmark of many cancers is their metabolic plasticity, as rewiring of cancer cells’ metabolic fluxes is essential for their proliferative and invasive agenda. With increasing understanding of these alterations, researchers are identifying features of tumor metabolism that can be used for novel diagnostic, prognostic, and therapeutic opportunities.
Highlighted Products
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()