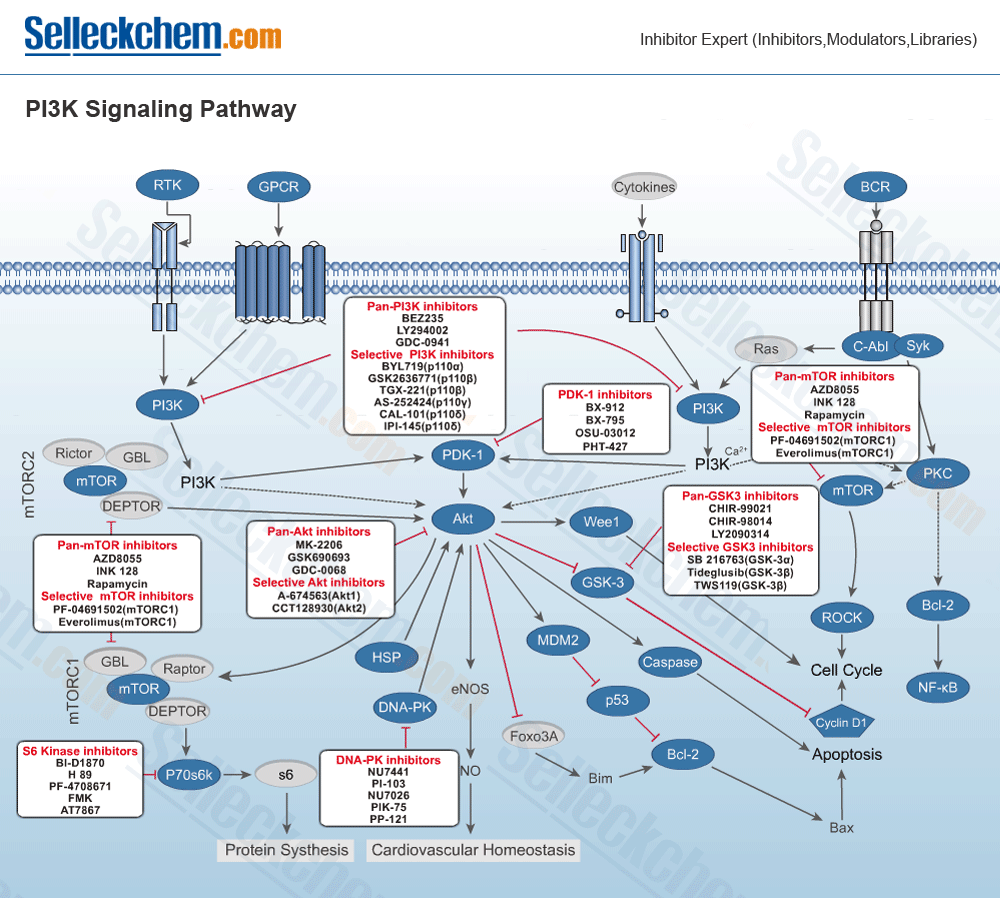
Selleck Chemicals provides products of the following targets:
- PI3K
- mTOR
- Akt
- GSK-3
- DNA-PK
- PDK-1
- AMPK
- ATM/ATR
- S6 Kinase
Various receptor tyrosine kinases (RTKs), such as human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), insulin-like growth factor receptor and vascular endothelial growth factor receptor, activate the growth and survive of cells in the manner of mobilizing the intracellular PI3K signaling pathway [12; 36]. The carcinogenic role of the PI3K pathway firstly came into the notice since mutation-induced abnormal cellular signaling was associated with malignant proliferation and growth [58]. Increasing evidence indicated that PI3K signaling pathway was usually found to be disordered in many kinds of cancers.
THE PI3K FAMILY MEMBERS
All PI3K family members have the ability to phosphorylate the 3-hydroxyl group of phosphoinositides [9]. They could be generally classified into three classes according to their primary structure, regulation, and in vitro lipid substrate specificity [34]. Class I PI3Ks are heterodimers containing a regulatory subunit and a catalytic subunit, which might be involved in malignant transformation in many different cancer types. The genes of PIK3R1, PIK3R2 and PIK3R3 encode the regulatory subunit, namely p85 (α, ß and γ) [38]. p110 is the catalytic subunit of class IA PI3K. The function of PI3K enzymes is mainly depended on it. There are four isoforms of p110: α, ß, γ and δ, which are encoded by the genes of PIK3CA, PIK3CB, PI3KCG and PIK3CD, respectively. Among the rest, p110-αis involved in the growth of cell and is the isoform which is often out of control under cancer state. In addition, class II PI3K enzymes are monomeric and involved in cell surface trafficking, whereas class III PI3K enzymes are implicated in the regulation of autophagy [38].
ACTIVITY CONTROL
The class IA PI3K enzyme complex is closely associated with phosphotyrosine residues on the intracellular membrane portion of RTKs. Under normal state, p85 locks the catalytic site of p110 subunit. Once the RTK was activated by its ligands, the catalytic site of p110 was expose. As a result, phosphatidylinositol 3,4,5-triphosphate (PIP3), as a second massager, was released at the cell membrane. This process could be regulated by phosphatase and tensin homolog (PTEN), which cut off the signal by metabolize PIP3 into PIP2. Therefor, lack of PTEN often lead to hyperactivation of PI3K pathway signaling. Another tumor suppressive factor named inositol polyphosphate 4-phosphatase type II (INPP4B) was found to negatively regulate the PI3K pathway by converting PIP2 into PIP [19]. Dual knockdown of INPP4B and PTEN in human epithelial cells has been shown to result in cellular senescence [19]. The PI3K pathway can also be mobilized by RAS, which could directly activate the catalytic subunit p110 [12].
MECHANISM OF ACTION
PIP3 recruits cellular proteins containing lipid-binding domains to cell membranes and binds to the pleckstrin homology domain in the N-terminal region of AKT. In this manner, PIP3 induces a conformational change in AKT revealing two amino acid, Thr308 and Ser473, and facilitate the full phosphorylation of Akt [47]. mTOR, also known as FKBP12-rapamycin-associated protein (FRAP), is a 280 kDa serine/threonine kinase downstream of Akt. mTOR is the nuclear catalytic subunit of two complexes: mTORC1 and mTORC2 [66]. mTORC1 is comprised of mTOR, Raptor, mLST8, and PRAS40 (a mTOR inhibitor). This complex presented classic features of mTOR as a nutrient or energy sensor and protein synthesis conditioner [16; 22; 29; 30; 46; 51]. The activated mTORC1 showed a negative feedback inhibition on PI3K signaling [61]. Different from mTORC1, activated mTORC2 could induce the phosphorylation of Akt at serine 473 and serving as a positive feedback on PI3K signaling cascade [53].
PI3K AND PAIN
Recently, several lines of evidence suggested the involvement of PI3K signaling cascades in the regulation of central and peripheral sensitization. The increased activation of Akt was often found in DRG and dorsal horn neurons [11; 48; 49; 57; 59; 60; 69]. Correspondingly, intrathecally injection of PI3K inhibitors suppressed nerve injury-induced (Xu et al., 2007) and chemical-induced [11; 48; 60] hyperalgesia. Furthermore, spinally inhibition of mTOR by rapamycin showed well anti-nociceptive effect in some animal pain models [5; 18; 28; 45; 50]. It suggested that PI3K pathway might be a potential target for the development of novel analgesics.
PI3K AND PROTEIN SYNTHESIS
What’s more, in young animals, a big mount of new protein synthesis are required in the nervous system development processes, like axon guidance and synapse formation [7; 8; 41; 67]. It was reported that BDNF induced translation of the Homer2 and GluR1 mRNAs in synaptoneurosomes could be well inhibited by using the specific PI3K–mTOR kinase inhibitors rapamycin and LY294002 [55]. Thus, the PI3K / Akt / mTOR related mRNA translation and protein expression seems to be more significant.
PI3K AND NEURAL PLASTICITY
On the other hand, in adult animal or human being, new protein synthesis also plays important roles in many aspects, such as the modulation of long-term synaptic plasticity (LTP), which is associated with learning and memory, as well as pain sensation mentioned above [6]. Specifically, it has been found that the local protein synthesis in neurosynapse is essential for the function and morphology of nervous system [27; 40]. In previous research, rapamycin, the specific inhibitor of mTOR, significantly suppressed the plastic changes in postmitotic neurons [10; 62]. It suggested the core role of mTOR in neural plasticity. In addition, the phosphorylation of 4EBP, the very downstream molecule of mTOR, was increased in rat hippocampus after spatial learning [42]. It suggested that PI3K/Akt/mTOR signaling-dependent mRNA translation is involved in the higher brain function.
PI3K AND ONCOLOGY
In recent decades, PI3K pathway was most frequently mentioned in the area of oncology for the reason that PI3K was almost involved in all developmental processes of tumor or cancer. The oncogenic transformation of cultured cells as well as the progression of a variety of tumors in vivo has been reported to be induced by mutations or overexpression of p110 isoforms. Mutations affecting the expression or function of p110 isoforms could induce oncogenic transformation [21; 37; 52]. The carcinogenic activity of p110α has been confirmed in ovarian cancers patient. Increased PIK3CA gene copies correlated with the overexpression of the p110α subunit led to an enhancive role of PI3 kinase [56]. Overexpression of hyperactive p110β derived the development of a intraepithelial neoplasia in the prostate of mice [33]. Importantly, Akt is probable to be the mediator of p110β-induced tumorigenesis [26]. There is also evidence revealed that the progression of multiple B-cell malignancies could be induced by a constitutive activation of p110δ [32].
PI3K AND SYSTEMIC IMMUNE
Chronic inflammation is a major cause of cancer. Much studies demonstrate that PI3Ks activity is essential in regulating chemokine production and the migration of leukocytes in immune response. For instance, in vivo research showed that p110γ is required to allow chemotactic migration of immune cells to inflammatory sites [39; 54]. Moreover, the release of inflammatory factors, like IL-8, Mip-1α, and Mip-1β, by neutrophils in response to immune stimulus requires the activity of p110δ complex [17]. PI3K/Akt pathway has also been reported to be related to the immune recognition of tumor cells. It was found that DAP10, the NKG2D-associated adapter protein in natural killer (NK) cells, could anchor to the p85 subunit of PI3K and lead to the activation of Akt pathway. This signaling cascades then strengthen the production of chemokine by NK cells [31; 63; 65].
In some cases, the PI3K pathway is also responsible for the escape of cells from immune system. A hyperactivation of Akt was found in a immune-resistant human papillomavirus type 16 (HPV-16) tumor cell line. It was thought to be responsible for the enhenced resistance of these cells to CD8 T-cell-mediated apoptosis [44]. Interestingly, cancer cells was found to suppress immune by some manners, including releasing immune-suppressive cytokines and chemokines, or inducing lymphocyte apoptosis [43; 64].
Due to the super activation of the PI3K signaling pathway in cancer cells, uncontrollable proliferation, survival and angiogenesis in various human malignancies had an opportunity to occur. Enormous efforts have been made for developing medicines targeting PI3K / Akt / mTOR pathway.
THE SPECIFIC INHIBITORS OF PI3K
Wortmannin and LY294002 are first-generation pan-PI3K inhibitors. Wortmannin, a furanosteroid metabolite of the fungi Talaromyces (Penicillium) wortmannii, is a specific inhibitor of PI3K. LY294002 was the first synthetic small molecule that reversibly inhibits the PI3K family members. Unfortunately, they have IC50 in micromolar range and displays similar potency for inhibiting the activity of Class I, II, and III PI3K members in vitro. In other words, they showed no selectivity for each PI3K isoforms. Furthermore, wortmannin or LY294002 displyed unfavorable pharmacokinetic properties and much toxicities in animals [4; 24; 35]. Thus it was failed to develop them into clinical drugs. However, these studies provide useful information of the PI3K signaling for the development of novel PI3K inhibitors.
In the following research works, isoform-selective PI3K inhibitors were reported. PI‑103 is able to inhibit both p110a and mTOR in glioma cells [15]. A Phase I study showed that a selective inhibitor of p110δ called CAL‑101 could be helpful with the treatment of hematologic malignancies in patients [25]. PX‑866, a pan-PI3K inhibitor, showed a mild side effect in part of the patients with solid tumors, such as squamous cell skin cancer and melanoma [2]. GDC0941, another pan-PI3K inhibitor, also displayed desirable anticancer effect in sarcoma, ovarian cancer and endometrial cancer patients [13].
THE SPECIFIC INHIBITORS OF AKT
Akt is another potential target for the therapy of cancer for the reason that Akt is one of the most important downstream molecule of PI3Ks. There are three isoforms of Akt: Akt1, Akt2 and Akt3. Perifosine, a PAN-Akt inhibitor, could bind to Akt and prevent Akt from binding to PIP3 and subsequent membrane translocation [23]. It has been in a Phase I clinical trial to treat solid tumors and lymphoma. A dual inhibitor of Akt1 and Akt2 named Akti‑1/2 showed potent antitumor activity in tumor xenograft models. MK2206, the analog of Akti‑1/2, is now in the Phase I clinical trial for the treatment of locally advanced or metastatic solid tumors and a Phase II study in advanced hepatocellular carcinoma [1; 3].
THE SPECIFIC INHIBITORS OF mTOR
As one of the key components of PI3K pathway, mTOR exists in two different complexes, mTORC1 and mTORC2. Rapamycin or sirolimus (Rapamune®; Wyeth, NJ, USA), which mainly inhibits the activity of mTORC1, presented well antitumor effect recently [14; 20]. However, for the bad solubility of rapamycin in aqueous solution, several rapamycin analogs such as Everolimus (RAD001/Afinitor®; Novartis, Basel, Switzerland) and temsirolimus (CCI‑779/ Torisel®; Wyeth) have been developed, which have been approved for the treatment of advanced renal cell carcinoma.
Under some conditions, inhibiting more than one target of PI3K pathway might lead to better effect. BEZ235, dual PI3K/mTOR inhibitor, inhibits Class I PI3K isoforms and mTOR kinase activity by binding to the ATP-binding pocket of PI3K. It has strong anti-proliferative effect on tumor xenografts and is now under Phase I/II clinical trials for breast cancer and endometrial cancer [1].
References
[1] NCI Clinical Trial website, wwwclinicaltrialsgov.
[2] A. Jimeno DSH, S. Hecker, R. Clement, R. Kurzrock, L. A. Pestano, A. Hiscox, R. A. Leos, D. L. Kirkpatrick, S. G. Eckhardt, R. S. Herbst. Phase I trial of PX-866, a novel phosphoinositide-3-kinase (PI-3K) inhibitor. J Clin Oncol 2009;27(15):3542.
[3] A. W. Tolcher TAY, I. Fearen, A. Taylor, C. Carpenter, A. T. Brunetto, M. Beeram, K. Papadopoulos, L. Yan, J. de Bono. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST). J Clin Oncol 2009;27(15):3503.
[4] Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 2005;115(10):2618-2624.
[5] Asante CO, Wallace VC, Dickenson AH. Formalin-induced behavioural hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol Pain 2009;5:27.
[6] Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci U S A 1996;93(24):13445-13452.
[7] Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 2002;110(2):223-235.
[8] Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 2001;32(6):1013-1026.
[9] Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002;296(5573):1655-1657.
[10] Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 1999;99(2):221-237.
[11] Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain 2010;149(2):243-253.
[12] Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010;28(6):1075-1083.
[13] D. D. Von Hoff PL, R. Tibes, G. Shapiro, G. J. Weiss, J. A. Ware, J. Fredrickson, K. E. Mazina, G. G. Levy, A. J. Wagner; Virginia G. Piper Cancer Center and TGen, Scottsdale, AZ. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J Clin Oncol 2009;27(15):3501.
[14] Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 2006;5(8):671-688.
[15] Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell 2006;9(5):341-349.
[16] Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 2001;294(5548):1942-1945.
[17] Fortin CF, Cloutier A, Ear T, Sylvain-Prevost S, Mayer TZ, Bouchelaghem R, McDonald PP. A class IA PI3K controls inflammatory cytokine production in human neutrophils. Eur J Immunol 2011;41(6):1709-1719.
[18] Geranton SM, Jimenez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci 2009;29(47):15017-15027.
[19] Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, Lin WM, Rameh L, Salmena L, Pandolfi PP, Cantley LC. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 2009;16(2):115-125.
[20] Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12(1):9-22.
[21] H. A. Dbouk HP, A. Fiser, and J. M. Backer. A biochemical mechanism for the oncogenic potential of the p110β catalytic subunit of phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences of the United States of America 2010;107(46):19897-19902.
[22] Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004;18(16):1926-1945.
[23] Hilgard P, Klenner T, Stekar J, Nossner G, Kutscher B, Engel J. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. Eur J Cancer 1997;33(3):442-446.
[24] Hu L, Zaloudek C, Mills GB, Gray J, Jaffe RB. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin Cancer Res 2000;6(3):880-886.
[25] I. W. Flinn JCB, R. R. Furman, J. R. Brown, T. S. Lin, C. Bello, N. A. Giese, A. S. Yu. Preliminary evidence of clinical activity in a phase I study of CAL-101, a selective inhibitor of the p1108 isoform of phosphatidylinositol 3-kinase (P13K), in patients with select hematologic malignancies. J Clin Oncol 2009;27(15s):3543.
[26] Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 2008;454(7205):776-779.
[27] Jiang C, Schuman EM. Regulation and function of local protein synthesis in neuronal dendrites. Trends Biochem Sci 2002;27(10):506-513.
[28] Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS One 2008;3(4):e1961.
[29] Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002;110(2):163-175.
[30] Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 2003;8(1):65-79.
[31] Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008;9(5):495-502.
[32] Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, Druker BJ, Puri KD, Ulrich RG, Giese NA. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011;117(2):591-594.
[33] Lee SH, Poulogiannis G, Pyne S, Jia S, Zou L, Signoretti S, Loda M, Cantley LC, Roberts TM. A constitutively activated form of the p110beta isoform of PI3-kinase induces prostatic intraepithelial neoplasia in mice. Proc Natl Acad Sci U S A 2010;107(24):11002-11007.
[34] Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol 1999;11(2):219-225.
[35] Lemke LE, Paine-Murrieta GD, Taylor CW, Powis G. Wortmannin inhibits the growth of mammary tumors despite the existence of a novel wortmannin-insensitive phosphatidylinositol-3-kinase. Cancer Chemother Pharmacol 1999;44(6):491-497.
[36] Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009;8(8):627-644.
[37] M. Sun PH, B. T. Hofmann, J. R. Hart, and P. K. Vogt. Cancer-derived mutations in the regulatory subunit p85α of phosphoinositide 3-kinase function through the catalytic subunit p110α. Proceedings of the National Academy of Sciences of the United States of America 2010;107(35):15547-15552.
[38] Markman B, Atzori F, Perez-Garcia J, Tabernero J, Baselga J. Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann Oncol 2010;21(4):683-691.
[40] Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol 2000;10(5):587-592.
[41] Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature 2002;417(6887):411-418.
[42] Mizuno M, Yamada K, Takei N, Tran MH, He J, Nakajima A, Nawa H, Nabeshima T. Phosphatidylinositol 3-kinase: a molecule mediating BDNF-dependent spatial memory formation. Mol Psychiatry 2003;8(2):217-224.
[43] Nicolini A, Carpi A. Immune manipulation of advanced breast cancer: an interpretative model of the relationship between immune system and tumor cell biology. Med Res Rev 2009;29(3):436-471.
[44] Noh KH, Kang TH, Kim JH, Pai SI, Lin KY, Hung CF, Wu TC, Kim TW. Activation of Akt as a mechanism for tumor immune evasion. Mol Ther 2009;17(3):439-447.
[45] Norsted Gregory E, Codeluppi S, Gregory JA, Steinauer J, Svensson CI. Mammalian target of rapamycin in spinal cord neurons mediates hypersensitivity induced by peripheral inflammation. Neuroscience 2010;169(3):1392-1402.
[46] O’Shea C, Klupsch K, Choi S, Bagus B, Soria C, Shen J, McCormick F, Stokoe D. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. EMBO J 2005;24(6):1211-1221.
[47] Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 2010;11(1):9-22.
[48] Pezet S, Marchand F, D’Mello R, Grist J, Clark AK, Malcangio M, Dickenson AH, Williams RJ, McMahon SB. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci 2008;28(16):4261-4270.
[49] Pezet S, Spyropoulos A, Williams RJ, McMahon SB. Activity-dependent phosphorylation of Akt/PKB in adult DRG neurons. Eur J Neurosci 2005;21(7):1785-1797.
[50] Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci 2007;27(51):13958-13967.
[51] Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene 2006;25(48):6373-6383.
[52] S. Kang AD, B. Vanhaesebroeck, and P. K. Vogt. Oncogenic transformation induced by the p110β, -γ, and -δ isoforms of class I phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences of the United States of America 2006;103(5):1289-1294.
[53] Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005;307(5712):1098-1101.
[54] Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 2000;287(5455):1040-1046.
[55] Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 2004;24(33):7366-7377.
[56] Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet 1999;21(1):99-102.
[57] Shi TJ, Huang P, Mulder J, Ceccatelli S, Hokfelt T. Expression of p-Akt in sensory neurons and spinal cord after peripheral nerve injury. Neurosignals 2009;17(3):203-212.
[58] Sugimoto Y, Whitman M, Cantley LC, Erikson RL. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A 1984;81(7):2117-2121.
[59] Sun R, Yan J, Willis WD. Activation of protein kinase B/Akt in the periphery contributes to pain behavior induced by capsaicin in rats. Neuroscience 2007;144(1):286-294.
[60] Sun RQ, Tu YJ, Yan JY, Willis WD. Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain 2006;120(1-2):86-96.
[61] Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, Kobayashi M. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol 2001;21(15):5050-5062.
[62] Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A 2002;99(1):467-472.
[63] Upshaw JL, Leibson PJ. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin Immunol 2006;18(3):167-175.
[64] Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 2006;16(1):3-15.
[65] Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science 1999;285(5428):730-732.
[66] Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124(3):471-484.
[67] Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, Dictenberg JB, Singer RH, Bassell GJ. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron 2001;31(2):261-275.
[69] Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 2004;24(38):8300-8309.