Nucleosomes are the fundamental repeating units of chromatin, comprised of a histone octamer wrapped by ~147bp DNA. A common focus of epigenetics research is the addition / removal of various post-translational modifications (PTMs) to histone tails, and how these marks impact downstream processes, such as transcription, cell division, and disease. Equally important, but less well understood, are the complexes that assemble, reformat, slide or eject nucleosomes in existing chromatin architecture. These nucleosome remodeling complexes are large, multi-protein structures, which are powered by the hydrolytic activity of associated ATPases1. Nucleosome remodeling is essential to the regulation of multiple pathways in cells, including transcription, genome replication, and DNA damage repair.
Exploring Chromatin Remodeling Substrates with Nucleosomes
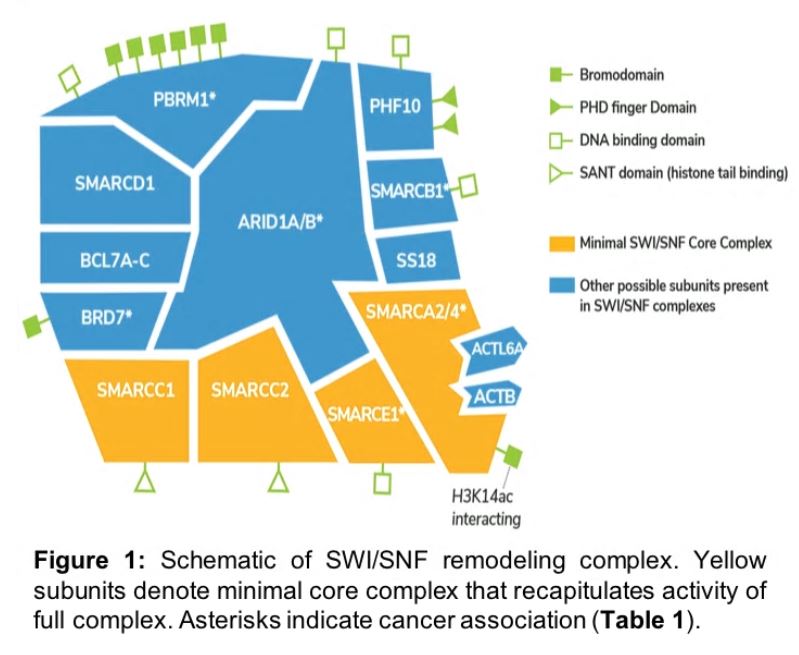
SWI/SNF nucleosome remodeling complexes
The most commonly studied chromatin remodeling complex is SWI/SNF, as it was the first linked to human cancer2, and has since been implicated in schizophrenia3, intellectual disability4, autism5, and cardiovascular development and disease6. The SWI/SNF complex contains ~15 protein subunits (Figure 1), including the mutually exclusive ATPases SMARCA2 and SMARCA4. The other proteins in the complex play diverse roles, interacting with specific DNA motifs, histone modifications, and transcriptional regulators, which localize nucleosome remodeling activity.
Targeting nucleosome remodelers for cancer therapy
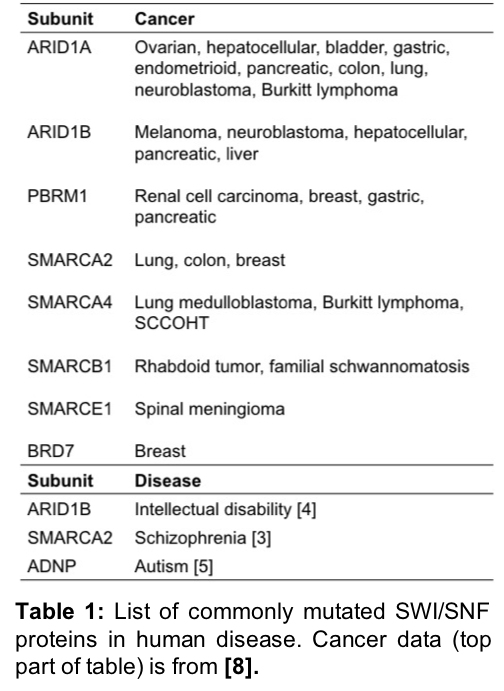
Remarkably, ~20% of all human cancers harbor mutations in SWI/SNF complex proteins7, with specific subunits of SWI/SNF correlating with distinct cancers8-12 (Table 1). For example, nearly 100% of patients with malignant rhabdoid tumors have mutations in SMARCB1, while approximately 46% of patients with renal cell carcinoma have mutations in PBRM11. Leveraging these mutant complexes for targeted therapeutic intervention is an ongoing area of cancer research1.
One potential route for treating SWI/SNF mutant cancers is selectively targeting the “residual” protein complexes. In the case of SMARCA4-deficient lung cancer, scientists found that cells were dependent on the remaining SMARCA2 ATPase for proliferation and survival; RNAi of SMARCA2 led to decreased proliferation and cancer cell death13-15. This phenomenon is referred to as synthetic lethality8, and can be exploited to selectively kill cancer cells, while sparing healthy, non-mutated tissues16, 17.
SMARCA2/4 Synthetic Lethality Is Dependent on the ATPase domain
There are two possible methods for leveraging synthetic-lethality in SWI/SNF mutant cancer drug development. The first is to target the catalytic domain of the remaining ATPase (e.g. SMARCA2 in SMARCA4-mutant lung cancer), blocking ATP-dependent nucleosome remodeling activity of residual complexes. The second approach is to identify inhibitors against the SMARCA2/4 bromodomain, which interacts with histone H3 lysine acetylation to localize remodeling activity on chromatin (Figure 1). The aim of these prospective treatments is to ablate SMARCA2 activity, replicating the synthetic lethality observed with RNAi, and inducing cancer-specific cell death.
Bromodomain inhibitors were already an active area of research following the success of the BET inhibitor JQ118, and are a relatively simple target compared to the complex ATPase domain. Thus, it was a logical first step to examine the utility of bromodomain inhibitors in modulating SMARCA2/4 synthetic lethality.
An elegant study by Vangamudi and colleagues19 tested the efficacy of the selective SMARCA2/4 bromodomain inhibitor PFI-3 in inducing cell death in SMARCA4-null lung cancer cell lines. Their results determined that, although PFI-3 was able to block GFP-tagged SMARCA2 from binding chromatin, it did not inhibit endogenous SWI/SNF complex localization, and had no effect on cancer cell proliferation or survival19. Additional testing of ATPase-null and bromodomain-null SMARCA2 constructs conclusively demonstrated that synthetic lethality requires inactivating the ATPase domain19. Further validating this theory, another study found that while targeting the SMARCA4 ATPase domain increased the clinical effectiveness of chemotherapy in breast cancer, blocking the bromodomain had no effect20.
However, screening and identifying ATPase inhibitors of nucleosome remodeling complexes has proven challenging. Existing assays primarily measure indirect byproducts of nucleosome repositioning (e.g. ATP hydrolysis), and as a result are prone to non-specific hits, often selecting for pan-ATPase inhibitors. Thus, new assays are desperately needed that directly measure nucleosome remodeling activity. Such screens require well-defined nucleosome substrates, which are expensive, difficult to produce, and often not readily available.
EpiDyne™: Next-generation nucleosomes remodeling substrates for HTS assay development
EpiCypher™ has pioneered the commercialization of recombinant nucleosome-based technologies for chromatin assay development, including our popular SNAP-ChIP™ and dCypherTM products / services. To accelerate the development of cancer therapeutics that target nucleosome remodelers, we have created our EpiDyne™ substrate product line. These recombinant nucleosome-based biochemical substrates are engineered for direct and rapid quantification of nucleosome remodeling activity.
Our objective with the line of EpiDyne products is to provide highly active enzymes (i.e. SMARCA2/4), defined, functional substrates, and optimized reagents and protocols that will enable drug discovery researchers to precisely target nucleosome remodeling complexes for therapeutic development. Currently, EpiDyne nucleosome remodeling substrates are available in a variety of formats, including FRET (see below), restriction enzyme accessibility, and DAM methyltransferase accessibility, providing maximum flexibility to meet individualized assay requirements.
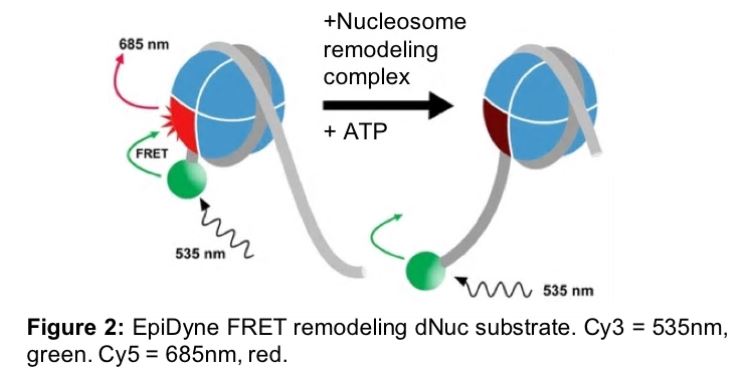
For example, EpiDyne-FRET is a one-step, no-wash method immediately compatible with high-throughput screening (HTS) applications. In this assay, a Cy5 labeled human histone octamer (Figure 2, in red) is wrapped with 5’ Cy3 labeled DNA (Figure 2, green ball). The Cy3 DNA label is adjacent to a TGGA-repeat region, refractory to nucleosome assembly, ensuring homogenous octamer preparations. Baseline FRET is at maximum, with the Cy3 DNA inducing fluorescence from the adjacent Cy5 histone (Figure 2, left). Following addition of a nucleosome remodeling complex, FRET is decreased, as the Cy3 DNA is no longer in close proximity to the Cy5-tagged histone (Figure 2, right).
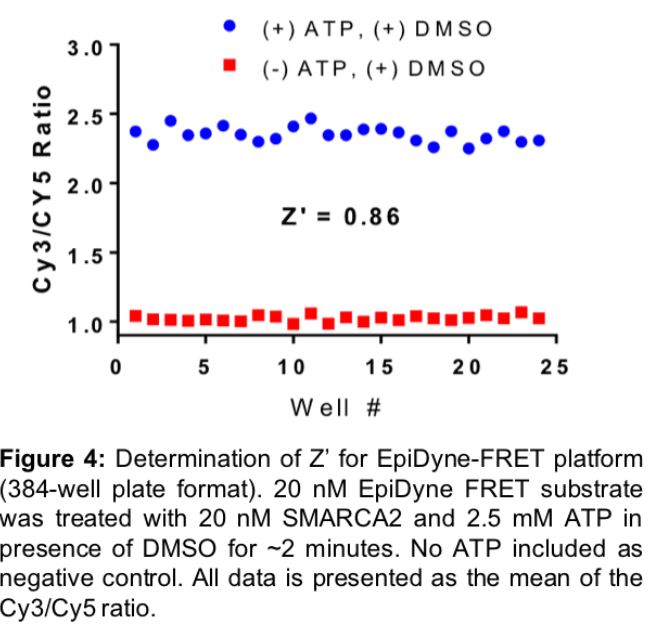
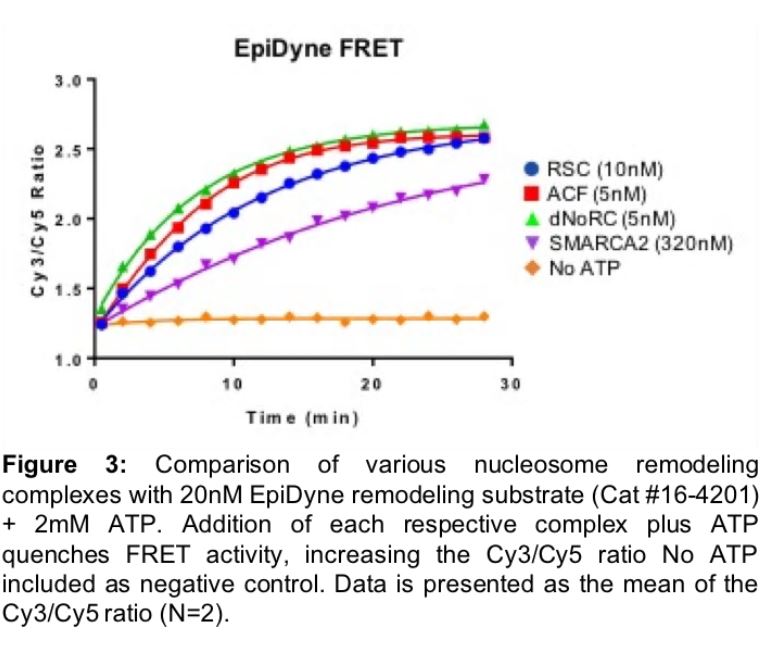
EpiCypher has optimized the EpiDyne-FRET system (Figure 3) and proven it to be a powerful assay for the rapid, direct detection of nucleosome repositioning. We have validated EpiDyne using a diverse set of ATP-dependent chromatin remodelers, including the Drosophila-derived ISWI enzymes ACF (Cat # 15-1013) and dNoRC, the yeast ATPase RSC, and the human SWI/SNF enzyme SMARCA2 (Figure 3). EpiDyne-FRET provides reliable and high-throughput readout for each enzyme, demonstrating its broad utility across several classes of chromatin remodelers. This is an important aspect of EpiDyne, as chromatin remodeling complexes act through a variety of methods, including the sliding activities of ISWI complexes (i.e. ACF, dNoRC) and the push / pull mechanisms common to SWI/SNF ATPases (i.e. RSC, SMARCA2)21. EpiDyne is also fully compatible with HTS, as Z’ factor studies indicate it is a reliable and consistent tool for the readout of nucleosome repositioning (Figure 4). EpiDyne’s unprecedented flexibility and HTS capabilities will help advance the development of drugs targeting multiple ATP-dependent chromatin remodeling complexes.
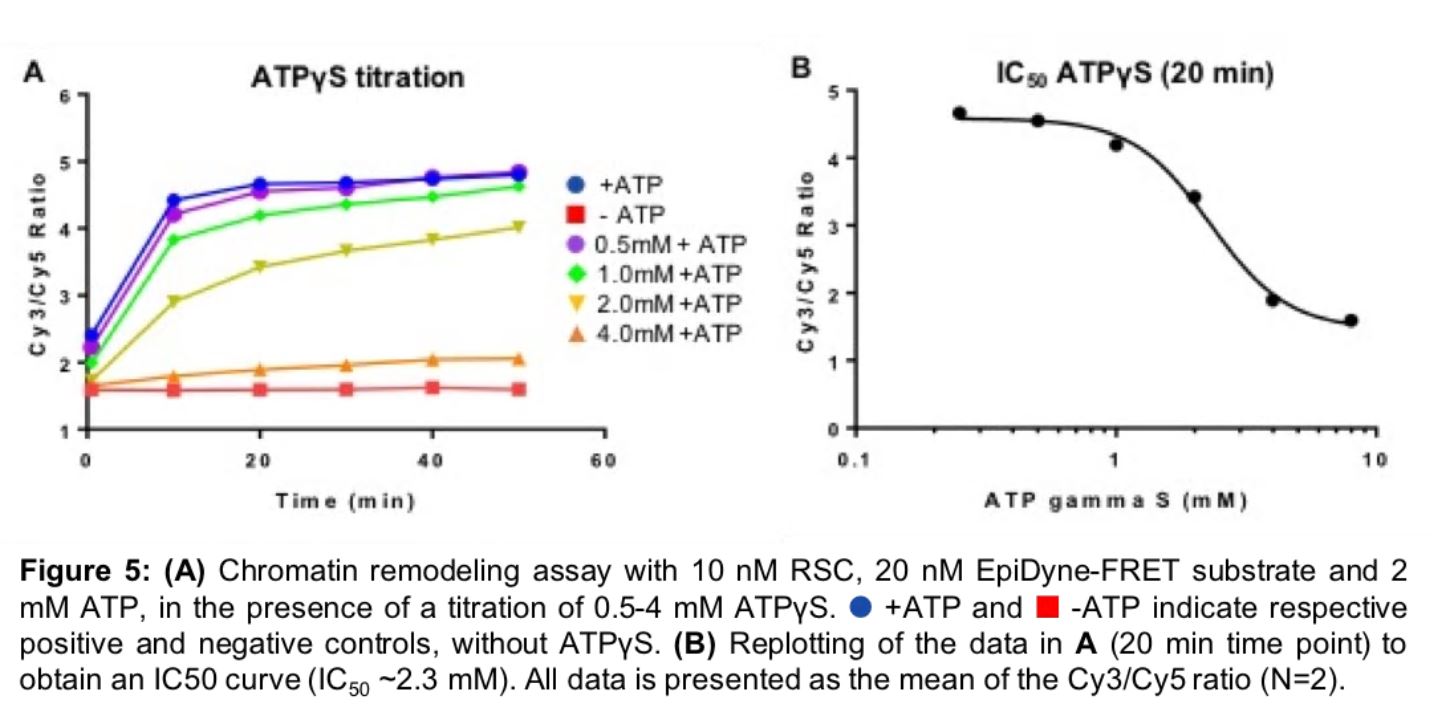
EpiDyne-FRET is also a highly sensitive and robust method for analyzing inhibition of nucleosome remodeling complexes. To model inhibitor dynamics we performed a titration of the non-hydrolyzable analog of ATP, ATPүS, against our EpiDyne-FRET substrate incubated with the SWI/SNF RSC enzyme. Increasing amounts of ATPүS successfully competed with ATP binding to RSC, resulting in nucleosome remodeling inhibition (Figure 5A). We determined the IC50 for ATPүS to be in the mM range (Figure 5B; ~2.3mM), further demonstrating the remarkable sensitivity of this assay and its application for drug development.
EpiDyne-FRET : An ideal HTS platform for nucleosomal remodeling inhibitors
EpiDyne-FRET fulfills all of the requirements for screening nucleosomal remodeling inhibitors, including 1) the use of a defined nucleosome substrate, 2) the assay readout directly reflects remodeling activity, and 3) provides a reliable and robust detection system. EpiDyne is a customizable platform, and EpiCypher can provide both reagents (i.e. dNuc substrates, ACF, SMARCA2, SMARCA4) and HTS services to fulfill project requirements. Of note, this product line is also customizable with our dNuc technology (i.e. histone PTMs can be incorporated into EpiDyne substrates that may improve or alter nucleosome remodeling activity). Inquire at info@stratech.co.uk for more information.
REFERENCES
1. Valencia AM, Kadoch C. Chromatin regulatory mechanisms and therapeutic opportunities in cancer. Nat Cell Biol, 2019. p. (PubMed PMID: 30602726)
2. Versteege I, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature, 1998. 394(6689): p. 203-6. (PubMed PMID: 9671307)
3. Koga M, et al. Involvement of SMARCA2/BRM in the SWI/SNF chromatin-remodeling complex in schizophrenia. Hum Mol Genet, 2009. 18(13): p. 2483-94. (PubMed PMID: 19363039)
4. Hoyer J, et al. Haploinsufficiency of ARID1B, a member of the SWI/SNF-a chromatin-remodeling complex, is a frequent cause of intellectual disability. Am J Hum Genet, 2012. 90(3): p. 565-72. (PubMed PMID: 22405089) (PMC3309205)
5. Helsmoortel C, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet, 2014. 46(4): p. 380-4. (PubMed PMID: 24531329) (PMC3990853)
6. Bevilacqua A, et al. SWI/SNF chromatin-remodeling complexes in cardiovascular development and disease. Cardiovasc Pathol, 2014. 23(2): p. 85-91. (PubMed PMID: 24183004) (PMC3946279)
7. Kadoch C, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet, 2013. 45(6): p. 592-601. (PubMed PMID: 23644491) (PMC3667980)
8. Helming KC, et al. Vulnerabilities of mutant SWI/SNF complexes in cancer. Cancer Cell, 2014. 26(3): p. 309-17. (PubMed PMID: 25203320) (PMC4159614)
9. Esteller M. Epigenetics in cancer. N Engl J Med, 2008. 358(11): p. 1148-59. (PubMed PMID: 18337604)
10. Kumar R, et al. Epigenomic regulation of oncogenesis by chromatin remodeling. Oncogene, 2016. 35(34): p. 4423-36. (PubMed PMID: 26804164)
11. Sperlazza J, et al. Depletion of the chromatin remodeler CHD4 sensitizes AML blasts to genotoxic agents and reduces tumor formation. Blood, 2015. 126(12): p. 1462-72. (PubMed PMID: 26265695) (PMC4573869)
12. Basta J, Rauchman M. The nucleosome remodeling and deacetylase complex in development and disease. Transl Res, 2015. 165(1): p. 36-47. (PubMed PMID: 24880148) (PMC4793962)
13. Oike T, et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res, 2013. 73(17): p. 5508-18. (PubMed PMID: 23872584)
14. Hoffman GR, et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc Natl Acad Sci U S A, 2014. 111(8): p. 3128-33. (PubMed PMID: 24520176) (PMC3939885)
15. Wilson BG, et al. Residual complexes containing SMARCA2 (BRM) underlie the oncogenic drive of SMARCA4 (BRG1) mutation. Mol Cell Biol, 2014. 34(6): p. 1136-44. (PubMed PMID: 24421395) (PMC3958034)
16. St Pierre R, Kadoch C. Mammalian SWI/SNF complexes in cancer: emerging therapeutic opportunities. Curr Opin Genet Dev, 2017. 42: p. 56-67. (PubMed PMID: 28391084)
17. Pulice JL, Kadoch C. Composition and Function of Mammalian SWI/SNF Chromatin Remodeling Complexes in Human Disease. Cold Spring Harb Symp Quant Biol, 2016. 81: p. 53-60. (PubMed PMID: 28408647)
18. Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature, 2010. 468(7327): p. 1067-73. (PubMed PMID: 20871596) (PMC3010259)
19. Vangamudi B, et al. The SMARCA2/4 ATPase Domain Surpasses the Bromodomain as a Drug Target in SWI/SNF-Mutant Cancers: Insights from cDNA Rescue and PFI-3 Inhibitor Studies. Cancer Res, 2015. 75(18): p. 3865-78. (PubMed PMID: 26139243) (PMC4755107)
20. Wu Q, et al. Targeting the chromatin remodeling enzyme BRG1 increases the efficacy of chemotherapy drugs in breast cancer cells. Oncotarget, 2016. 7(19): p. 27158-75. (PubMed PMID: 27029062) (PMC5053639)
21. Cairns BR. Chromatin remodeling: insights and intrigue from single-molecule studies. Nat Struct Mol Biol, 2007. 14(11): p. 989-96. (PubMed PMID: 17984961) (PMC2788559)