Abcalis® anti-SARS-CoV-2 antibodies are high quality recombinant monoclonal primary antibodies
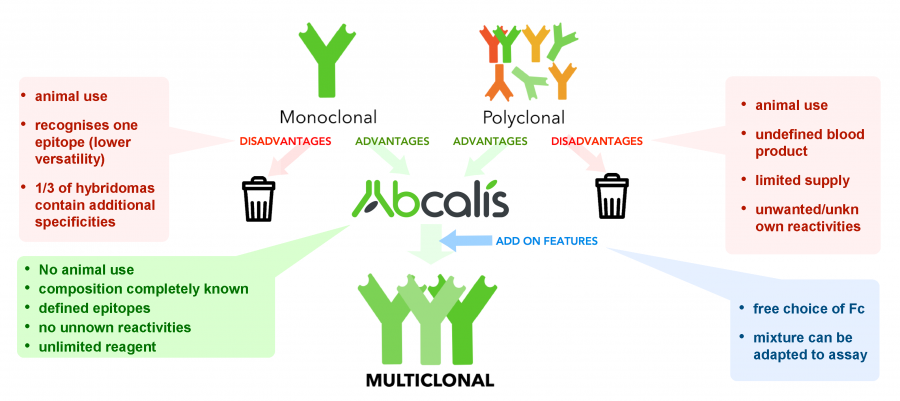
Abcalis’ product portfolio now also features anti-SARS-CoV-2 recombinant (sequence defined) monoclonal antibodies. We believe that recombinant primary antibodies can deliver a significant improvement over the current standard testing procedure of utilizing RT-PCR testing. Given that RT-PCR tests are complex, cost-intensive and time-consuming, the tests are not truly suitable for the real-time control of epidemiological measures due to the time delay from taking the sample until getting a result, which also makes them unsuitable for mass market real-time diagnostics for point-of-care (POC) testing (e.g. medical practices, hospitals, etc.).
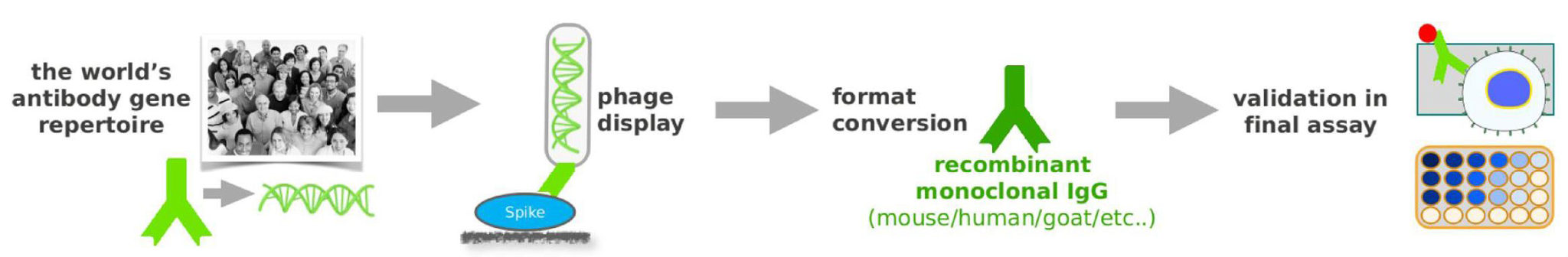
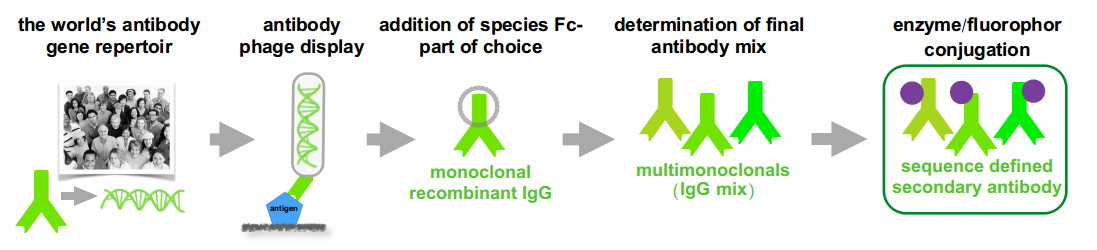
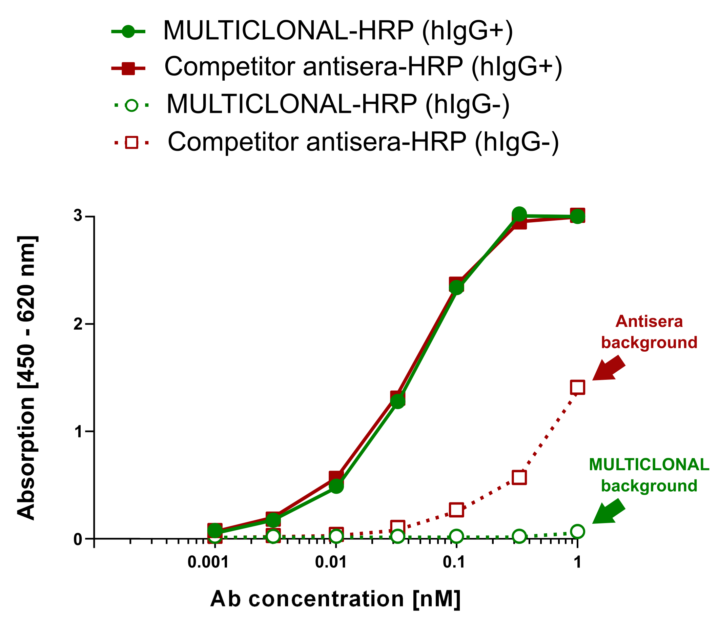
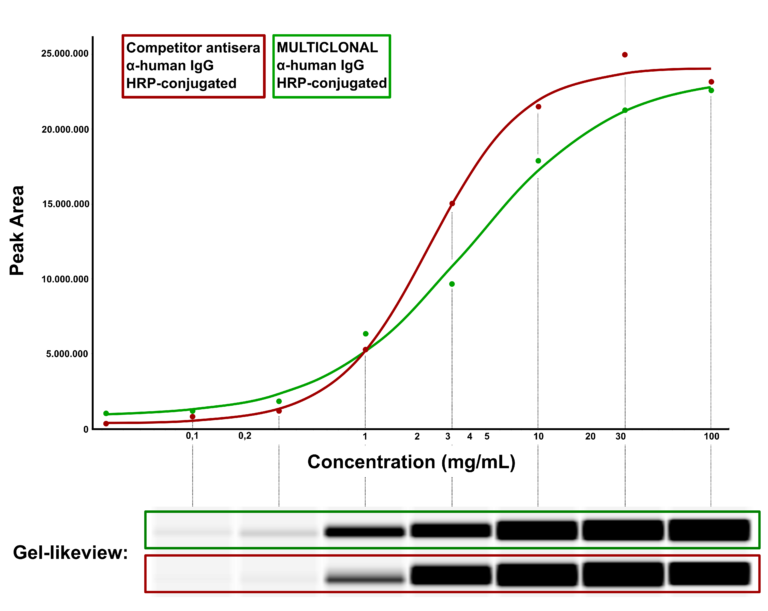
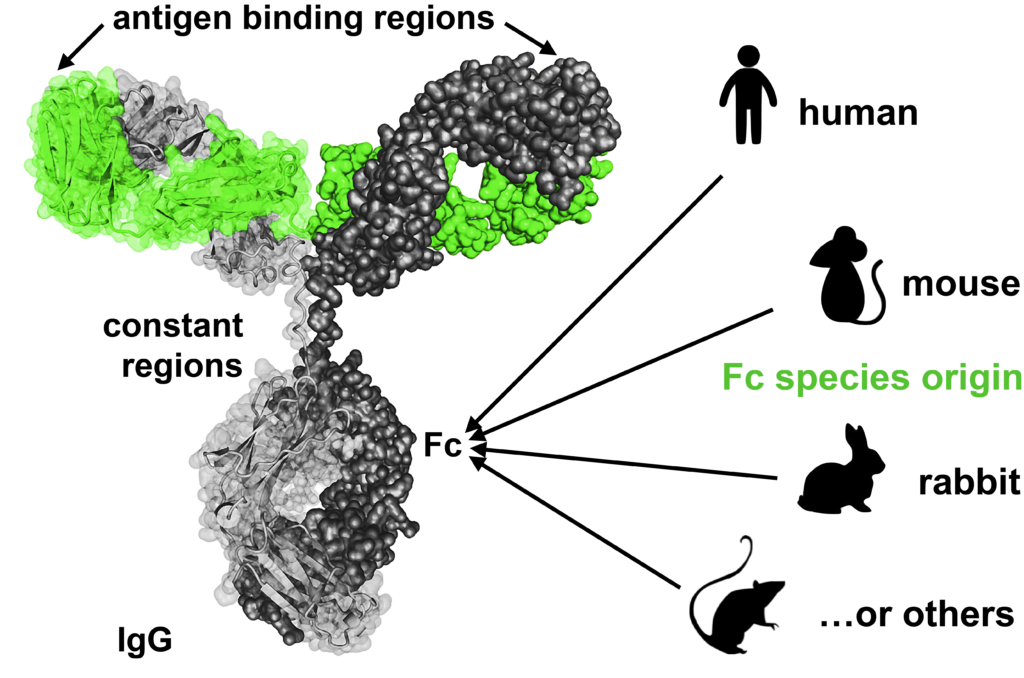
Hence Abcalis’ approach for recombinant primary antibodies to be used in antigen based rapid tests. See below how we select and validate our anti-SARS-CoV-2 antibodies: