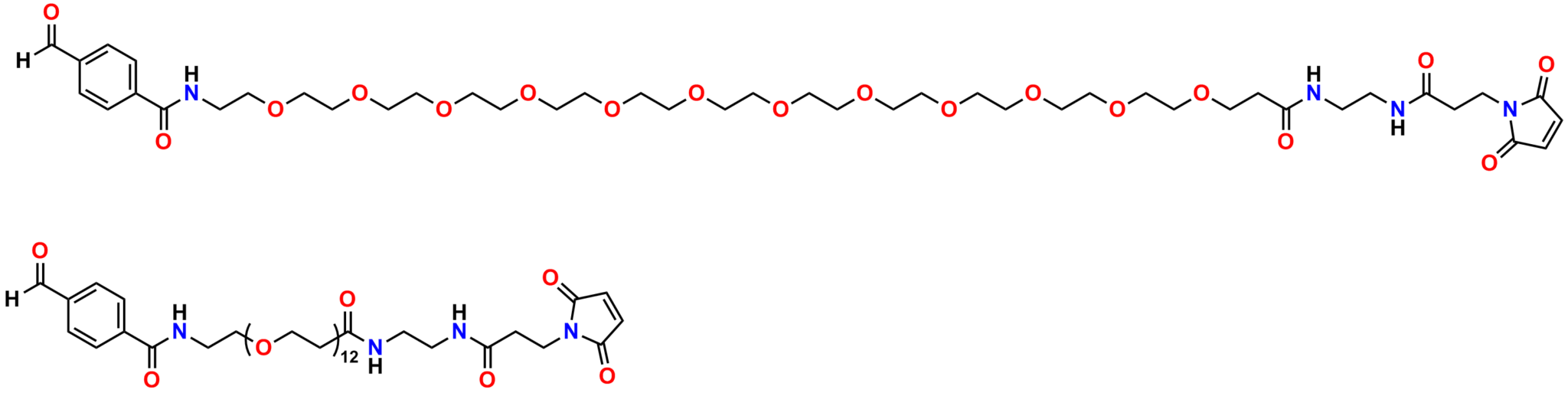
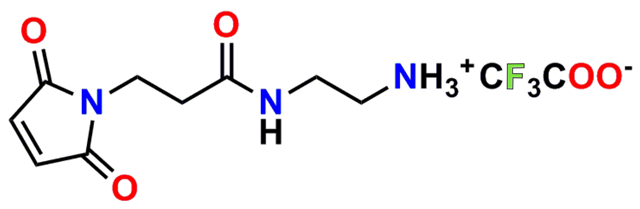
Finally, Quanta BioDesign offers product number 10177, MPS-EDA∙TFA (Figure 16), a short, non-PEGylated maleimide crosslinker product. This product crosslinks a sulfhydryl group with a carboxylic acid, or it can be used as a building block with discrete PEG to form a new crosslinker having a longer spacer between the maleimide group and the PEG linker.
The links below go to the maleimide crosslinker products on this website’s Other Thiol Reactive product page discussed in this section.
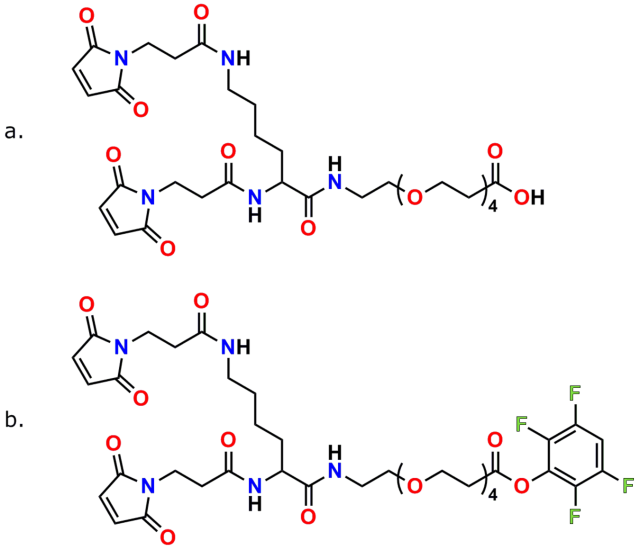
PN10630, bis-MAL-Lysine-dPEG®4-acid
PN10631, bis-MAL-Lysine-dPEG®4-TFP ester
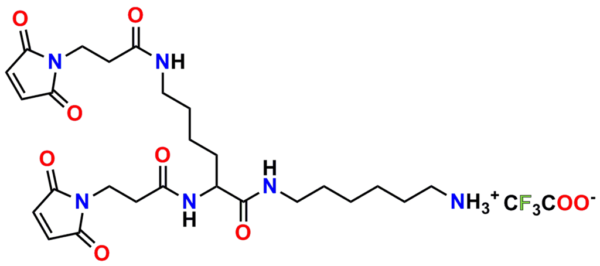
PN10232, Bis-Maleimide Amine, TFA salt
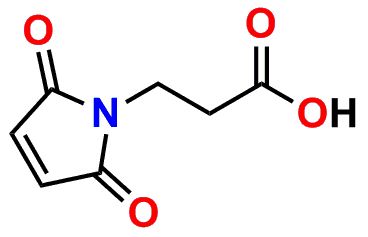
PN10323, MPS-acid
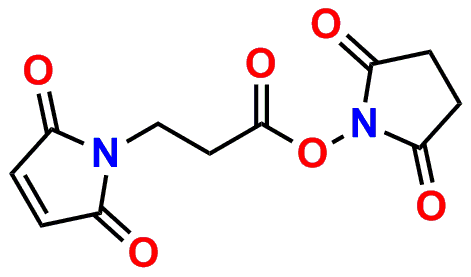
PN10217, MPS
PN10177, MPS-EDA∙TFA
Conclusion
Quanta BioDesign offers many varied crosslinkers with maleimide functional groups on one or more ends of linear and multi-armed discrete PEG (dPEG®) products. These crosslinkers enable the conjugation of thiols with amines or thiols with other thiols. Numerous product applications exist for these crosslinkers, including antibody-drug conjugates (ADCs), fragment-drug conjugates (FDCs), protein-complex proximity analysis, FRET applications, modification of thiol-functionalized surfaces, supramolecular materials construction, and many more.
We say that our maleimide crosslinker products are “marvelous” because our dPEG® technology makes them so. Discrete PEG products improve the hydrophilicity of poorly water-soluble products and reduce the immunogenicity of potentially antigenic compounds. Because these products are single molecular weight products, the analysis of bioconjugates is simplified compared to traditional, non-uniform (i.e., polydispersed) PEG products.
We invite you to consider our maleimide-dPEG® crosslinkers for your research and development applications. Our products are used in diagnostic and therapeutic applications globally and have been featured in hundreds of peer-reviewed scientific publications and thousands of patents over the past 20+ years. Try our dPEG® products and discover the dPEG® difference!
References
[1] Hermanson, G. T. Chapter 3, The Reactions of Bioconjugation. In Bioconjugate Techniques; Academic Press: New York, NY, 2013; pp 229-258. Greg’s book is the industry-standard reference in the science and technology of bioconjugation, and we sell it on our website. If you don’t have a copy, you should, and you can purchase it by clicking this link now!
[2] Bragg, P. D.; Hou, C. Subunit Composition, Function, and Spatial Arrangement in the Ca2+– and Mg2+-Activated Adenosine Triphosphatases of Escherichia Coli and Salmonella Typhimurium. Archives of Biochemistry and Biophysics 1975, 167(1), 311-321. https://doi.org/10.1016/0003-9861(75)90467-1.
[3] Lomant, A. J.; Fairbanks, G. Chemical Probes of Extended Biological Structures: Synthesis and Properties of the Cleavable Protein Cross-Linking Reagent [35S]Dithiobis(Succinimidyl Propionate). Journal of Molecular Biology 1976, 104(1), 243-261. https://doi.org/10.1016/0022-2836(76)90011-5.
[4] Li, T.; Takeoka, S. Enhanced Cellular Uptake of Maleimide-Modified Liposomes via Thiol-Mediated Transport. Int. J. Nanomed. 2014, 9(1), 2849-2861. https://doi.org/10.2147/IJN.S58540
[5] Che, J.; Okeke, C. I.; Hu, Z.-B.; Xu, J. DSPE-PEG: A Distinctive Component in Drug Delivery System. Curr. Pharm. Des. 2015, 21(12), 1598-1605.
[6] Wang, J.; Zhang, R.-Y.; Wang, Y.-C.; Chen, X.-Z.; Yin, X.-G.; Du, J.-J.; Lei, Z.; Xin, L.-M.; Gao, X.-F.; Liu, Z.; Guo, J. Polyfluorophenyl Ester-Terminated Homobifunctional Cross-Linkers for Protein Conjugation. Synlett 2017, 28(15), 1934-1938. https://doi.org/10.1055/s-0036-1590974.
[7] Partis, M. D.; Griffiths, D. G.; Roberts, G. C.; Beechey, R. B. Cross-Linking of Protein by ω-Maleimido Alkanoyl N-Hydroxysuccinimido Esters. J Protein Chem 1983, 2(3), 263-277. https://doi.org/10.1007/BF01025358.
[8] Quanta BioDesign, personal observations with TFP ester reactions, 2019.
[9] Belowich, M. E.; Stoddart, J. F. Dynamic Imine Chemistry. Chem. Soc. Rev. 2012, 41(6), 2003-2024. https://doi.org/10.1039/C2CS15305J.
[10] Wendeler, M.; Grinberg, L.; Wang, X.; Dawson, P. E.; Baca, M. Enhanced Catalysis of Oxime-Based Bioconjugations by Substituted Anilines. Bioconjugate Chem. 2014, 25(1), 93-101. https://doi.org/10.1021/bc400380f.
[11] Larsen, D.; Kietrys, A. M.; Clark, S. A.; Park, H. S.; Ekebergh, A.; Kool, E. T. Exceptionally Rapid Oxime and Hydrazone Formation Promoted by Catalytic Amine Buffers with Low Toxicity. Chem. Sci. 2018, 9(23), 5252-5259. https://doi.org/10.1039/C8SC01082J.
[12] Kölmel, D. K.; Kool, E. T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117(15), 10358-10376. https://doi.org/10.1021/acs.chemrev.7b00090.
[13] Baxter, E. W.; Reitz, A. B. Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents. In Organic Reactions; American Cancer Society, 2004; pp 1-714. https://doi.org/10.1002/0471264180.or059.01.
[14] Fondo, M.; Corredoira-Vázquez, J.; GarcíaDeibe, A. M.; Sanmartín-Matalobos, J. An Easy Approach to Obtain Alcohol-Amines by Reduction of Alcohol Functionalized Imines. Proceedings 2019, 9(1), 2. https://doi.org/10.3390/ecsoc-22-05697.
[15] Billman, J. H.; Diesing, A. C. Reduction of Schiff Bases with Sodium Borohydride. J. Org. Chem. 1957, 22(9), 1068-1070. https://doi.org/10.1021/jo01360a019.
[16] Hutchins, R. O.; Hutchins, M. K.; Crawley, M. L.; Mercado-Marin, E. V.; Sarpong, R. Sodium Cyanoborohydride. In Encyclopedia of Reagents for Organic Synthesis; American Cancer Society, 2016; pp 1-14. https://doi.org/10.1002/047084289X.rs059.pub3.
[17] Tadanier, J.; Hallas, R.; Martin, J. R.; Stanaszek, R. S. Observations Relevant to the Mechanism of the Reductive Aminations of Ketones with Sodium Cyanoborohydride and Ammonium Acetate. Tetrahedron 1981, 37(7), 1309-1316. https://doi.org/10.1016/S0040-4020(01)92446-9.
[18] Abdel-Magid, A. F.; Carson, K. G.; Harris, B. D.; Maryanoff, C. A.; Shah, R. D. Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures1. J. Org. Chem. 1996, 61 (11), 3849-3862. https://doi.org/10.1021/jo960057x.
[19] Gribble, G. W.; Abdel-Magid, A. F. Sodium Triacetoxyborohydride. In Encyclopedia of Reagents for Organic Synthesis; American Cancer Society, 2007. https://doi.org/10.1002/9780470842898.rs112.pub2.
[20] Kim, T. H.; Jiang, H. H.; Lee, S.; Youn, Y. S.; Park, C. W.; Byun, Y.; Chen, X.; Lee, K. C. Mono-PEGylated Dimeric Exendin-4 as High Receptor Binding and Long-Acting Conjugates for Type 2 Anti-Diabetes Therapeutics. Bioconjugate Chem. 2011, 22(4), 625–632. https://doi.org/10.1021/bc100404x.
[21] Scheer, J. M.; Sandoval, W.; Elliott, J. M.; Shao, L.; Luis, E.; Lewin-Koh, S.-C.; Schaefer, G.; Vandlen, R. Reorienting the Fab Domains of Trastuzumab Results in Potent HER2 Activators. PLOS ONE 2012, 7(12), e51817. https://doi.org/10.1371/journal.pone.0051817.