ATS FAQ’s
I was wondering if you could elaborate on why the Streptavidin-ZAP product recommends to be used at an equimolar ratio with the targeting reagent, when it is capable of binding up to four biotins/molecule?
It’s a question we get asked sometimes and it’s a good question.
You are correct that streptavidin is capable of binding up to 4 biotin molecules. However, when we created streptavidin-ZAP with the purpose of being a modular way of creating targeted toxins, we learned that the best general rule to follow was using a equimolar reaction. In theory, it is a 1:1 ratio of targeting molecule to streptavidin-ZAP, where we are most likely seeing an average of 1:1, but there is also the possibility of mixed ratios.
The amount of publications using the equimolar approach gave the desired results whether they were using a small biotinylated peptide or whole IgG. You’ll notice that depending on the MW of your biotinylated targeting agent, the amount of streptavidin-ZAP needed for the experiment can vary drastically and through in-house characterization, the equimolar approach still worked best.
Another reason we recommend a 1:1 ratio is based on our experience with our other secondary conjugates. It may be intuitive to think that using a higher dose of targeting agent would induce more cell death, but we found the opposite effect, where the excess, un-reacted targeting agent competed with the conjugated material for surface binding sites, which in turn decreased the amount of saporin being delivered. We have a publication (PMCID: PMC8952126 ) that also describes this observation.
Once you’ve created a baseline using the equimolar protocol and are more accustomed to how streptavidin-ZAP works in your application, please contact us if you feel more optimization is needed. It will be easier to help trouble-shoot when we are all working off the same protocol.
Which type of RIP is saporin?
I read on your website that, “There are two types of RIPs: type I, which are much less cytotoxic due to the lack of the B chain and type II, which are distinguished from type I RIPs by the presence of the B chain and their ability to enter cells on their own.” In the IT-27 Streptavidin-ZAP product, which type of saporin is there? Is it both type I and type II because the saporin is purified from the plant, or is it one specific type only in the product.
All saporin molecules are Type I ribosome-inactivating proteins. We only use saporin. An example of a Type II RIP is ricin, which can enter a cell on its own and has been used throughout history as a method of assassination.
Streptavidin-ZAP is streptavidin attached to saporin. On its own it has no way to get inside a cell. By mixing Streptavidin-ZAP with a biotinylated molecule that is recognized on the cell surface, the resulting conjugate is able to bind and internalize saporin into a cell. Once inside saporin inactivates the ribosomes which causes cell death.
Fab-ZAP Final Concentration
When using any of your Fab-Zap product line, the recommended final concentration is 4.5 nM. Is this based on experiments you have done? I question if at 4.5 nM my primary antibody will be saturated with Fab-ZAP secondary conjugate?
Yes, the 4.5 nM concentration is what we use to quality-control test our Fab-ZAP conjugates and why we recommend it in the literature. We also recommend only titrating your primary antibody. The 4.5 nM of Fab-ZAP should be enough to saturate your primary antibody. If you have a test of ~10 nM of primary antibody and you experience less cell death than ~1 nM, this will indicate “antibody competition” (i.e., your primary antibody is not saturated).
Neuropeptide Toxins
What are neuropeptide-toxins and how do they work?
Neuropeptide-toxin conjugates are made up of the ribosome-inactivating protein, saporin, coupled to a naturally-occurring or synthetically-modified neuropeptide such as Substance P or dermorphin. The conjugate has binding specificity similar to the native, unconjugated neuropeptide. When the neuropeptide binds to its cognate receptor, the conjugate is internalized. Once inside the target cell within an endosome, the neuropeptide and saporin separate and some of the saporin translocates into the cytoplasm where it catalytically inactivates ribosomes resulting in cell death.
Are neuropeptide-toxins effective suicide transport agents?
The general answer to this question is not currently known. However, in the instance of intrathecally injected dermorphin-SAP (Cat. #IT-12), the evidence does NOT favor suicide transport of the neuropeptide-toxin conjugate. When supramaximal doses of dermorphin-SAP (750 ng) are injected into the lumbar subarachnoid space of adult rats, less than 1% of lumbar dorsal root ganglion cells show evidence of saporin activity. This is in spite of the fact that many of these neurons express the targeted mu opioid receptor on their central terminals in the superficial dorsal horn of the spinal cord. This assertion is based on analysis of over 16,000 neurons from dorsal root ganglia in six rats.
Mab-ZAP binds to Fc portion of mouse IgG
Does Mab-ZAP (Cat. #IT-04) bind to the FC portion of mouse IgG?
The antibody used to create our Mab-ZAP (IT-04), will react with whole molecule mouse IgG, which includes the Fc portion and the two antigen binding Fab portions.
FabFc-ZAP cross-reaction with another species
Can your FabFc-ZAP human (Cat# IT-65) bind to the Fc portion of another species, such as mouse IgG? It looks like it binds to mouse IgG in our assay.
The antibody used to create our FabFc-ZAP Human (IT-65), can react with the Fc (gamma) portion of human IgG heavy chain and should not react with the Fab portion of human IgG. However, there could be minimal cross-reaction with mouse, horse, or bovine serum proteins, and it is possible to see cross-reaction with immunoglobulins from other species.
Custom Saporin Conjugations
We recently spoke to you about performing a custom saporin conjugation using our antibody. Is 0.09% azide in PBS in the antibody stock acceptable?
There are a number of dialysis steps within the conjugation protocol that will ultimately remove the azide from your antibody solution. So as long as your antibody will be happy in PBS without azide during the procedure, sending the material in 0.09% azide is fine. The final conjugate will be returned to you in PBS, sterile-filtered, without azide.
In general, how many saporin molecules are incorporated per antibody? Can we test this by HPLC?
We aim for 2-2.5 moles of saporin per mole of antibody. You should be able to see differences in HPLC between your antibody with one vs. two vs. three saporins attached, however we will provide you with a saporin molar ratio and a product that has had free saporin and free antibody removed from the final conjugate.
Dosage of Fab-ZAP for antibody concentration
Is the dosage of Fab-ZAP always enough for any level of antibody concentration?
The 4.5 nM dosage of Fab-ZAP is the recommended concentration. We do not typically see unspecific killing at 4.5 nM on most cell lines. If the concentration of Fab-ZAP is increased, it may undergo bulk-phase endocytosis and kill cells indiscriminately. A lower concentration of Fab-ZAP may lead to antibody competition, resulting in a lack of killing of cells at the highest concentration of antibody.
Recommended ratio between Fab-ZAP dosage and antibody concentration
Is there a recommended ratio between Fab-ZAP dosage and antibody concentration?
A recommended good starting point is 4.5 nM of Fab-ZAP, with a titration of the antibody starting at a concentration of 10 nM.
Fab-ZAP number of replications
Each concentration is suggested to perform 6 replications, can it be adjusted more or less in practice?
Yes, the assay design is meant to be a thorough approach but can be adjusted by the user. We recommend 6 replications based on our 96-well plate template design. The concentration of Fab-ZAP is 4.5 nM in the suggested protocols.
Fab-ZAP number of replications
Each concentration is suggested to perform 6 replications, can it be adjusted more or less in practice?
Yes, the assay design is meant to be a thorough approach but can be adjusted by the user. We recommend 6 replications based on our 96-well plate template design. The concentration of Fab-ZAP is 4.5 nM in the suggested protocols.
Detecting the targeted antibody in supernatant
Can Fab-ZAP detect the targeted antibody still in supernatant?
As long as there is nothing in the supernatant inhibiting the reactivity of Fab-ZAP, it should work. We do not typically recommend this, but in theory it should be possible. I would be cautious of this approach based off of the presumed lack of established concentration of antibody.
DMSO% for peptide and Streptavidin-ZAP
Instead of performing the reaction between our biotinylated peptide and Streptavidin-ZAP at the initially provided concentration of Strep-ZAP (20 µM), is it OK if the reaction is done at a 10-fold more dilute concentration? This request is to ensure we don’t have any solubility problems with our very tricky lipophilic peptide. Our protocol would be to first dilute Streptavidin-ZAP to 2 µM with PBS and then add the peptide in DMSO (10% final), and store the aliquoted resulting 1.82 µM solution?
In regards to your question, while keeping in mind your solubility concerns, we suggest that you:
- Proceed with diluting the Streptavidin-ZAP to 2 uM with PBS as you suggest, BUT, only react the amount of Streptavidin-ZAP necessary for the next step.
- Store the undiluted and unreacted Streptavidin-ZAP at -80°C until you’re ready for more conjugate.
We understand the solubility of the peptide is a concern, and rightfully so. However, we also do not want to compromise the Streptavidin-ZAP during storage, considering its value.
ZAP Internalization Kit Concentrations
e have your ZAP internalization kit and I have a question regarding the concentrations used in the cytotoxicity assay. The Hum-ZAP used in the assay (mentioned in the PDF protocol) is 4.5 nM and the target agent was 10 nM to 1 fM. Is there a stoichiometric relation between Hum-ZAP and the target agent concentrations?
To answer your question simply, yes, there is a stoichiometric relation between a secondary conjugate and the targeting agent.
If I use higher concentrations of the target antigen, then should I also increase the concentration of Hum-ZAP?
It may be intuitive to think that using a higher dose of primary antibody induces a higher amount of cell death, but as seen in the attached figure, at the highest concentration of 192-IgG (10 nM = Log -8) there is a lessened amount of killing, at a 25-fold lower concentration, as compared to the antibody. The explanation for this is that, at the higher concentrations of primary antibody, there is more unconjugated 192-IgG and fewer 192-IgG+Fab-ZAP complexes. The free 192-IgG then out-competes the conjugates for cell surface binding sites which, in turn, decreases the amount of Saporin being internalized, hence less cell death.
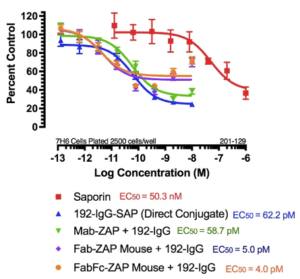
Using Kit Controls
Your targeted toxin kits come with different controls. I’m not sure of the best way to use them; there is included unconjugated antibody, unconjugated saporin, and a control conjugate, mouse IgG-SAP. Should I use them all in the same experiment or for different purposes?
For mouse IgG-containing conjugates, the ideal control is Mouse IgG-SAP (Cat. # IT-18). Mouse IgG-SAP — that is, saporin conjugated to mouse IgG — that has no specific antigen for targeting is the best control. Unconjugated saporin is still considered a second good control, useful in cases where down-regulation by the antibody is a concern.
What about for the peptide toxins?
We have produced Blank-SAP as a control for the peptide ligand toxins. Blank-SAP (Cat. #IT-21) is a peptide that has the usual common amino acids that are found in peptide neurotransmitters, but arranged in a sequence that is random and not detected in homology searches. So, it should never find an amenable receptor. This is quite an important control; the peptide ligand toxins are often delivered directly to tissue, and there are cases in which there will be no toxicity or non-specific toxicity.
One of the best uses we have seen for Blank-SAP has been in: Bugarith K et al. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146(3):1179-1191, 2005.
As any journal reviewer will tell you, it’s very important to document the specificity, and with Blank-SAP as a control, you can definitively show that toxicity is due to proper targeting, rather than non-specific cytotoxicity. This should provide the information needed so the reviewer doesn’t have to make you go back and document specificity with further experimental work!

Which secondary ZAP conjugate should I use?
I’ve been looking at your secondary conjugates and want to see if my targeting agent is specific to certain cells. Which secondary conjugate should I use?
It depends on two factors: 1) the type of assay you want to use, and 2) the kind of targeting agent you want to use.
For in vitro assays, in particular, internalization assays, you can use any of the ZAP Antibody Internalization Kits (Z-Kits that include all the materials necessary to test your targeting agent).
For the Antibody Internalization Kits, you use your primary antibody and select the appropriate secondary antibody species, depending on the isotype of your primary antibody — e.g. for a human antibody, use Hum-ZAP (Cat. #KIT-22-Z), Fab-ZAP human (Cat. #KIT-51-Z), FabFc-Human (Cat. #KIT-65-Z), Hug-M-ZAP (Cat. #KIT-43-Z), or Fab-ZAP Hug-M (Cat. #KIT-78-Z).
Or you can biotinylate your antibody and use Biotin-Z Antibody Internalization Kit (Cat. #KIT-27-Z).
For in vivo applications, it depends on the kind of targeting agent you want to use. Regardless of whether you use an antibody, peptide or ligand, you will need to biotinylate the material first. ATS offers a biotinylation service that is efficient and economical.
If you are using a biotinylated peptide, you will use a kit that includes the appropriate control — Streptavidin-ZAP Peptide Kit (Cat. #KIT-27-B).
If you are using a biotinylated antibody, you will use the Streptavidin-ZAP Antibody Kit that includes the appropriate antibody species control: BIgG-SAP Human (Cat. #KIT-27-Ahu), BIgG-SAP Mouse (Cat. #KIT-27-Amu), BIgG-SAP Rabbit (Cat. #KIT-27-Arb), BIgG-SAP Rat (Cat. #KIT-27-Art). Select the kit that matches the species of your biotinylated primary antibody.
Related: ZAP Secondary Conjugates
If I use higher concentrations of the target antigen, then should I also increase the concentration of Hum-ZAP?
It may be intuitive to think that using a higher dose of primary antibody induces a higher amount of cell death, but as seen in the attached figure, at the highest concentration of 192-IgG (10 nM = Log -8) there is a lessened amount of killing, at a 25-fold lower concentration, as compared to the antibody. The explanation for this is that, at the higher concentrations of primary antibody, there is more unconjugated 192-IgG and fewer 192-IgG+Fab-ZAP complexes. The free 192-IgG then out-competes the conjugates for cell surface binding sites which, in turn, decreases the amount of Saporin being internalized, hence less cell death.

Saporin Safety
Over the years, ATS has frequently been asked about Saporin’s safety for use in the lab as well as when used clinically. Residual awareness of alternate Ribosome-Inactivating Proteins (RIPs) and ‘toxins’ such as Ricin have caused some researchers new to the use of RIPs to question the belief that Saporin is safe. Unlike Type 2 RIPs (such as Ricin), Type I RIPs, like Saporin have no binding chain and consequently no means of entering the physiological space necessary for the protein to act as a toxin. The following is a review of safety in handling and potential toxicity within the human body for systemic events not related to normal research applications of Saporin conjugates, including Substance P-Saporin (SP-SAP), which is a therapeutic under development for the treatment of chronic pain.
The acute LD50 for saporin in mice (25 g) is 6.8 mg/kg;[1] that would translate in humans (75 kg) to 510 mg! A concentration of about 100 nM is the threshold to see even a vague hint of saporin toxicity. In human blood, that would correspond to 24 mg injected systemically into a person. The fermentation process to produce recombinant saporin has a titer of 2 mg/L meaning that the production broth itself contains no more than 67 nM concentration of saporin. Furthermore, the final protein concentrations from production batches of recombinant Saporin used in our drug are 4 mg/ml, meaning 6 mL of final material would need to accidentally end up in a human before the ‘hint of toxicity’ threshold would potentially be met.
The toxicology studies of SP-SAP contained within ATS’s IND prior to the current human Phase I clinical trial evaluated effects related to the intended method of administration, intrathecal local injection. SP-SAP is not expected to ever be a self-administered therapy, so the effects of gross off-target events, such as accidental auto-injection, swallowing, spillage, or immersion were not considered.
The table below[2] highlights antibody-saporin conjugates approved by the FDA for Phase I/II clinical trials in humans. The therapeutics listed below were administered intravenously and imply what the FDA accepted as non-toxic levels of saporin-based conjugates in these studies.
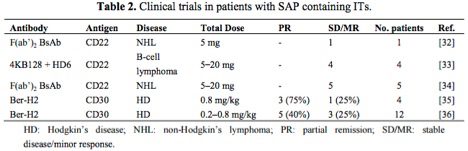
Looking more closely at the study by French et al.,[3] several milligrams of antibody conjugate were repeatedly injected into human patients under a FDA regulated clinical trial and peak serum levels tested, demonstrating rapid clearing of saporin from the system.
As a company that specializes in Saporin, our two-plus decades of experience working with the protein in research, preclinical, and clinical environments has taught us that with minimal standard laboratory precautions users are not at any real risk of toxic effects. Even our CSO, after 30+ years of working with Saporin exhibits undetectable levels of Saporin antibodies in his blood!
References:
- Thorpe PE et al. An immunotoxin composed of monoclonal anti-thy 1.1 antibody and a ribosome-inactivating protein from Saponaria officinalis: potent antitumor effects in vitro and in vivo. J Natl Cancer Inst 75:151-159, 1985.
- Polito L et al. Immunotoxins and other conjugates containing saporin-s6 for cancer therapy. Toxins (Basel) 3(6):697-720, 2011.
- French RR et al. Response of B-cell lymphoma to a combination of bispecific antibodies and saporin. Leuk Res 20(7):607-17, 1996.
Safety of the Toxin
Our QA group wants to know about the safety of the toxin in your conjugates. What precautions should we take in handling saporin products?
Saporin is a Type 1 ribosome-inactivating protein (RIP), due to its N-glycosidase activity, from the seeds of Saponaria officinalis. It was first described by Fiorenzo Stirpe and his colleagues in 1983 in an article that illustrated the unusual stability of the protein.[1] Among the RIPs are some of the most toxic molecules known, including ricin and abrin (the latter is the poison preferred by the characters in the movie The Blue Lagoon). These toxins contain a second protein strand that inserts the RIP into a cell, making it able to enzymatically inactivate the ribosomes, shutting down protein synthesis and resulting in cell death and eventually causing death of the victim.
Saporin does not possess a cell-binding chain[2] and has no method of internalization without a targeting agent to escort it into a cell. It is this fact that also adds to the safety of its use in the lab. Autoclaving or exposure to 0.2 M NaOH is sufficient to decontaminate material that has been in contact with Saporin and its conjugates. The LD50 for Saporin in mice is 4-8 mg/kg.[3] With an average person, let’s say 75 kg, that would be more than what you might have in your freezer, let alone be able to inject in yourself. Targeted Saporin, if targeted to a human epitope, should be handled more carefully, but due to logistics, it’s difficult to imagine an effect. Hundreds of articles in the scientific literature (search “Saporin” in Pub Med) have demonstrated tremendous specificity in targeting neuronal cells with many different Saporin conjugates and by many different scientists.
References
- Stirpe F et al. Ribosome-inactivating proteins from the seeds of Saponaria officinalis L. (soapwort) of Agrostemma githago L. (corn cockle) and of Asparagus officinalis (asparagus) and from the latex of Hura crepitans L. (sandbox tree). Biochem J 216:617-625, 1983.
- Barthelemy I et al. The expression of saporin, a ribosome-inactivating protein from the plant Saponaria officinalis, in Escherichia coli. J Biol Chem 268(9):6541-6548, 1993.
- Stirpe F et al. Hepatotoxicity of immunotoxins made with saporin, a ribosome-inactivating protein from Saponaria officinalis. Virchows Arch B Cell Pathol Incl Mol Pathol 53(5):259-271, 1987.
Retrograde Transport
I’m trying to find out if enough Anti-DBH-SAP will be retrogradely transported and taken up by non targeted sympathetic neurons by bulk fluid-phase endocytosis. Does saporin become degraded after it kills the neuron or does it enter the extracellular matrix?
It is very unlikely that a targeted toxin such as Anti-DBH-SAP is freed from the targeted neuron in a meaningful condition. There has never been a reported identification of a targeted toxin, functionally or not, after it has eliminated its targeted neuron. Current evidence indicates that effective suicide transport agents undergo endocytosis at nerve terminals followed by retrograde axonal transport of the endocytic vesicles containing the toxin. Experiments using vincristine have shown that the retrograde axonal transport of suicide transport toxins utilizes the fast transport system (microtubules). However, it is not known what determines whether or not a specific toxin-ligand undergoes axonal transport after internalization.
in vitro Cytotoxicity Assays
For in vitro cytotoxicity assays, could you tell me: 1) whether you incubate primary with your Saporin secondary for a specific amount of time prior to cell addition, and 2) do you use a single concentration of secondary per well or a primary:secondary ratio — like 1:2 or 1:4?
The primary antibody should be incubated with the ZAP product for 20 min prior to addition to the cells. Internalization often happens so quickly that you would lose some efficacy due to the antibody being bound and internalized prior to the ZAP product complexing with the primary. We do recommend maintaining a constant 5 nM (~ 45 ng/well) concentration of the ZAP product in the well and titrating your primary only. This way the EC50 you generate will be the EC50 of the primary antibody with all else held constant. The best starting concentration for your primary antibody is 10-100 nM in the well.
Targeted Toxin Format
I ordered a targeted toxin. Will it come in powder form? How do I re-dissolve it?
Our Saporin conjugate products are all provided in sterile PBS solution within a concentration range of 0.5 – 3 mg/ml. Saporin is an extremely safe ‘toxin’ to handle in standard laboratory environments when in solution for several reasons. Solutions in general are easier to corral and keep contained than powders and consequently are less likely to accidentally end up on an individual’s skin, tongue, or in one’s eyes. As a lyophilized product, Saporin would also be present at an extremely high concentration such that there is cause for concern should it contact the body of the user in any way. Lastly, our Saporin conjugates have historically required dilution prior to use for both in vitro and in vivo procedures. As such, it is much easier to ensure the amount of material you, as a customer, are receiving and the subsequent dilution is accurately adjusted to your desired concentration when providing these products already in solution. If upon receiving a Saporin conjugate you believe the product to be lyophilized or in a powder form, please contact us immediately, prior to opening the vial.
