Microneedle-assisted genome editing: A transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders.
The immune system plays a vital role in maintaining the health of all the tissues of the body. It reacts to invaders, such as microorganisms, foreign substances, or cancer cells, triggering inflammation to attack invaders. Usually, the immune system reaction protects the body and aids healing. However, sometimes an immune system reaction is misdirected at healthy tissues and causes intense inflammation and damage. Hypersensitivity (allergic) and inflammatory skin disorders are caused by immune system reactions that involve the skin.
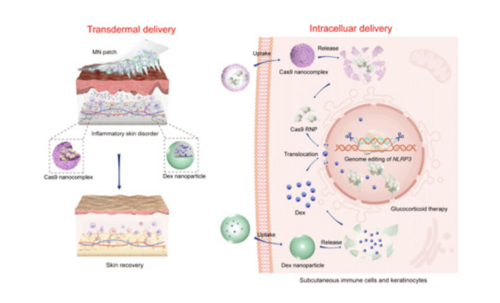
Inflammatory skin disorders (ISDs) represent one of the most persistent diseases characterized by the activation of innate and adaptive immune responses by producing proinflammatory cytokines. Activation of inflammatory reactions is caused by supramolecular complexes of inflammatory proteins called inflammasomes. Among various subtypes of inflammasomes, nod-like receptor family, pyrin domain–containing 3 (NLRP3) has been associated with a variety of inflammatory and autoimmune skin conditions psoriasis and atopic dermatitis (AD). Its activation is relevant to several allergic stimuli in the inflammatory processes.
Topical administration of glucocorticoids is widely endorsed as the first-line anti-inflammatory treatment in patients with AD or psoriasis. However, most patients often show inadequate response to glucocorticoid therapy in the long-term treatment, primarily due to glucocorticoid resistance. Evidence indicates that the overexpression of CASP-1 (encoding caspase-1) and its activator’s up-regulation, the NLRP3 inflammasomes, can cause glucocorticoid resistance. It modulates the functional glucocorticoid receptor’s cellular levels in its transactivation domain and diminishes glucocorticoid transcriptional effects to increase glucocorticoid resistance in immune-related cells. Researchers have begun to target NLRP3 inflammasomes inhibition to alleviate inflammatory responses. For effective delivery, it is essential to consider the skin barriers that prevent the inhibitors’ entry at different levels. Thus, the efficient transdermal delivery of a highly specific, potent, and direct NLRP3 inhibitor seems to be an ideal promising strategy to combat ISDs.