Selection of fluorophores depends on:
- Instrument set‑up. Examples include availability of light sources, filter sets and detection systems.
- Degree of colour separation desired for multiple labelling. For example, to achieve good colour separation from Alexa Fluor® 488, choose a shorter wavelength emitting fluorophore such as DyLight 405 and/or longer wavelength‑emitting fluorophores such as Rhodamine Red‑X, Alexa Fluor® 594, or Alexa Fluor® 647.
- Sensitivity required. For example, Alexa Fluor® 488 is brighter than FITC.
The following fifteen fluorescent dye conjugates (Figure 2, Table 1) are currently available from Jackson ImmunoResearch. They cover the most commonly used excitation sources and filter sets from blue to infrared emissions.
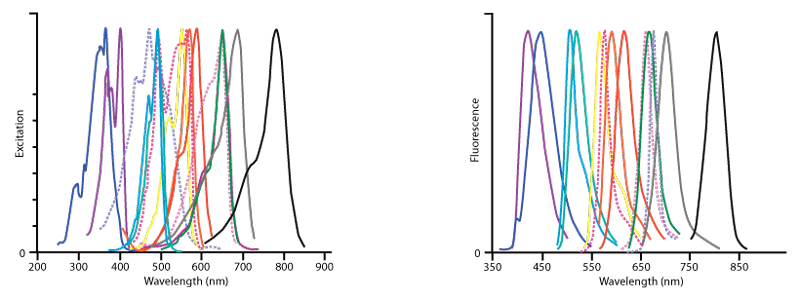
Figure 2. Excitation (left) and emission (right) spectra of all fluorophores offered by Jackson ImmunoResearch.The conjugates in order of increasing wavelength of fluorescence are DyLight 405 (violet), AMCA (dark blue),Cy2 (light blue), Alexa Fluor® 488 (blue‑green), FITC (hidden behind Alexa Fluor® 488), Cy3 (yellow), R‑PE (dark pink, dotted), Rhodamine Red‑X (orange), Alexa Fluor® 594 (red), Allophycocyanin (pink, dotted), Cy5 (green), Alexa Fluor® 647 (purple‑hidden behind Cy5), PerCP (lavender, dotted), Alexa Fluor® 680 (grey) and Alexa Fluor® 790 (black) . This figure illustrates the relative shape and position of each fluorophore in the peak region of its excitation and fluorescence emission following conjugation to antibodies. Quantitative comparisons should not be made since peak heights have been normalised. All spectra were obtained with an M‑Series spectrofluorometer system from Photon Technology International, Inc.
Alexa Fluor® Fluorescent Dyes
Alexa Fluor® fluorescent dyes are widely recognised as superior fluorescent dyes available for conjugation. They are highly water soluble and remain fluorescent from pH 4 to pH 10. The detection level of any fluorophore‑antibody conjugate depends on brightness and photostability of the dye; antibody activity, specificity and cross‑reactivity; and the optimal moles of dye per mole of antibody. These parameters have been researched for each dye conjugate to optimise the level of antibody detection and minimise background.
| Fluorophore | Excitation Peak | Emission Peak (nm) |
| DyLight 405 | 400 | 421 |
| Aminomethylcoumarin, AMCA | 350 | 450 |
| Cyanine, Cy2 | 492 | 510 |
| Alexa Fluor® 488 | 493 | 519 |
| Fluorescein, FITC/DTAF | 492 | 520 |
| Indocarbocyanine, Cy3 | 550 | 570 |
| R‑Phycoerythrin, R‑PE | many, 488 | 580 |
| Rhodamine Red‑X, RRX | 570 | 590 |
| Alexa Fluor® 594 | 591 | 614 |
| Allophycocyanin, APC | many, 650 | 660 |
| Alexa Fluor® 647 | 651 | 667 |
| Indodicarbocyanine, Cy5 | 650 | 670 |
| Peridinin‑Chlorophyll‑protein, PerCP | many, 488 | 675 |
| Alexa Fluor® 680 | 684 | 702 |
| Alexa Fluor® 790 | 792 | 803 |
Cyanine dyes (Cy2, Cy3, and Cy5)
Among currently available fluorescent dyes, the cyanine dyes are better able to withstand the harsh dehydration and embedding conditions required for mounting sections in non‑polar plastic media, such as DPX and Permount. The cyanine dyes are brighter in the non‑polar environment than in an aqueous medium, resulting in less acquisition time than other dyes in the confocal microscope. The advantage of using plastic mounting media is better long‑term storage for re‑examination and retrieval of data at a later date.
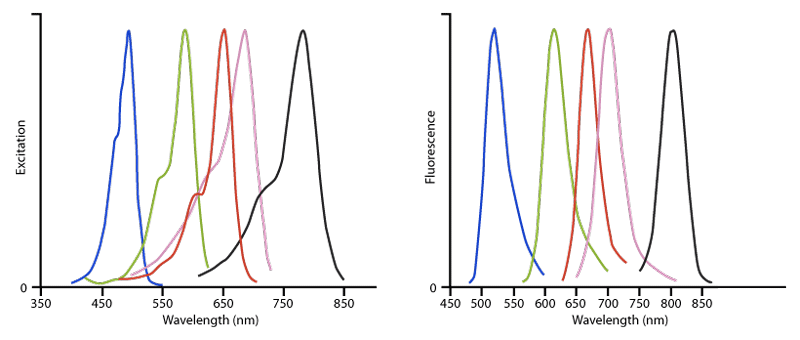
Figure 3. Excitation (left) and Emission (right) spectra of Alexa Fluor® conjugated affinity‑purified secondary antibodies, streptavidin and purified proteins. The dyes are Alexa Fluor® 488 (blue), Alexa Fluor® 594 (green), Alexa Fluor® 647 (red), Alexa Fluor® 680 (pink) and Alexa Fluor® 790 (black). This figure illustrates the relative shape and position of each fluorophore conjugate in the peak region of its excitation and fluorescence emission. Quantitative comparisons should not be made since peak heights have been normalised. All spectra were obtained with an M‑Series spectrofluorometer system from Photon Technology International, Inc.
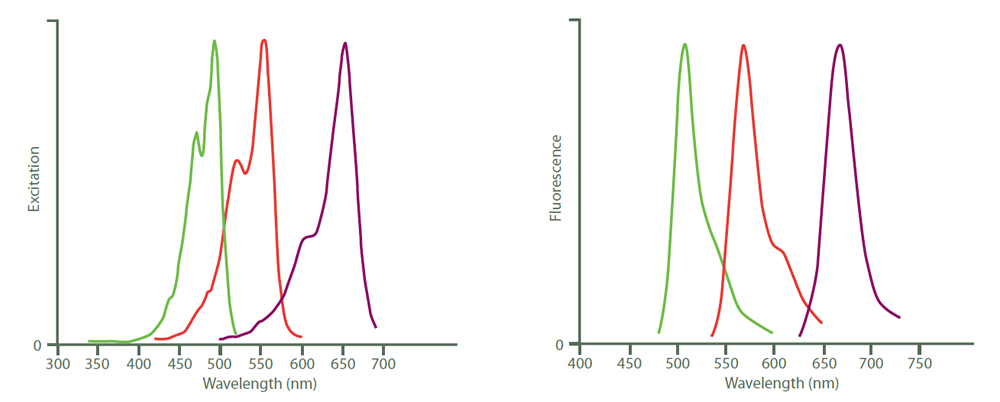
Figure 4. Excitation (left) and emission (right) spectra of Cy2 (green), Cy3 (red) and Cy5 (purple). Peak heights were normalised after the spectra were obtained with an M‑series spectrofluorometer system from Photon Technology International, Inc.
Fluorescent Dyes in Order of Increasing Emission Wavelength
A brief description of the characteristics of fluorophores found in this catalogue is found below. The fluorophores are listed in order of increasing emission wavelength (Figure 2 and Table 1).DyLight 405‑conjugated secondary antibodies are excited maximally at about 400 nm and fluoresce with a peak at about 421 nm (Figure 2 and Table 1). They are very bright and photostable, but their optimal use is limited to confocal microscopes equipped with a 405 nm laser and a 420 nm emission filter. Under these conditions, it is possible to perform effective 4‑colour imaging with good colour separation, good photostability and high sensitivity. The combination of DyLight 405, Alexa Fluor® 488, Rhodamine Red‑X and Alexa Fluor® 647 provides for maximum colour separation (Figure 5). Another 4‑colour dye combination, which may be equally effective but has slightly less colour separation, is DyLight 405, Alexa Fluor® 488, Cy3 and Alexa Fluor® 647. DyLight 405 is not recommended for use in epifluorescence microscopes, nor is it recommended for flow cytometry, because emission filters generally used in flow cytometers are not optimal for DyLight 405. DyLight 405 conjugates are the best choice for blue‑fluorescing secondary antibodies in multi‑colour labelling protocols. See tables of Whole IgG Affinity‑Purified Antibodies, Streptavidin and Purified Proteins from Normal Serums for all DyLight 405 conjugates.
AMCA (Aminomethylcoumarin Acetate) conjugates absorb light maximally around 350 nm and fluoresce maximally around 450 nm (Figure 2 and Table 1). For fluorescence microscopy, AMCA can be excited with a mercury lamp and observed using a UV filter set. Since blue fluorescence is not well detected by the human eye, AMCA Conjugated secondary antibodies should be used only with the most abundant antigens in multiple‑labelling experiments. Ways of improving the visibility of AMCA include dark adapting the eyes, using fluorite instead of glass objectives, avoiding mounting media that absorb UV light (such as plastic‑based media) and capturing photographic images with blue‑sensitive film or CCD cameras. AMCA fades rapidly in conventional epifluorescence and confocal microscopy and therefore it should be used with mounting media containing an anti‑fading agent such as n‑propyl gallate.
For flow cytometry, AMCA can be excited with a mercury lamp or with a water‑cooled argon ion laser which emits some lines in the UV. AMCA has been used mostly for multiple labelling since there is minimal fluorescence overlap with green‑fluorescing dyes and little or no overlap with longer wavelength‑emitting fluorophores. Applications for multiple labelling with this probe include both immunofluorescence microscopy and flow cytometry. AMCA is not suggested for single labelling in one‑photon microscopy because of its relatively weak signal and rapid fading. However, AMCA has been found to be a bright and photostable dye for 2‑photon microscopy. In one‑photon microscopy DyLight 405 is a better choice for multiple labelling.
Cy2 conjugates have maximum absorption/excitation at 492 nm and fluoresce with a peak around 510 nm in the green region of the visible spectrum (Figure 2 and Table 1) like FITC conjugates (520 nm), but they are more photostable and less sensitive to pH changes than FITC. However, for mounting in aqueous media we recommend Alexa Fluor® 488 as the preferred green‑fluorescing dye because it is brighter and more photostable than Cy2, FITC and DyLight 488. A further disadvantage of using Cy2 with aqueous mounting media is its sensitivity to p‑phenylenediamine, an anti‑fading agent found in some commercial mounting media, which results in weak and diffused fluorescence after storage of stained slides. The main advantage of Cy2 conjugates is increased fluorescence in plastic mounting media. Cy2 is available conjugated to a limited selection of antibodies.
Alexa Fluor® 488‑conjugated antibodies absorb light maximally at 493 nm and fluoresce with a peak around 519 nm (Figure 2 and Table 1). In aqueous mounting media they are brighter than FITC, Cy2 and DyLight 488. Alexa Fluor® 488 conjugates are recommended for maximum sensitivity for all immunofluorescence procedures requiring a green‑fluorescing dye, except for protocols that include mounting in plastic mounting media.
FITC (Fluorescein isothiocyanate) is the form of fluorescein used for conjugation to all of our antibodies and purified proteins, with the exception of streptavidin. Fluorescein conjugates absorb light maximally at 492 nm and fluoresce maximally at 520 nm (Figure 2 and Table 1). Although less bright than other green‑fluorescing dyes, FITC is still a widely used fluorophore due to its long history. The major disadvantage of fluorescein is its rapid photobleaching (fading), which can be mitigated by the use of an anti‑fading agent in the mounting medium. A better choice for many applications involving FITC is Alexa Fluor® 488 because it is brighter and more photostable.
DTAF (Dichlorotriazinylamino fluorescein) is another form of fluorescein, with excitation and emission peaks identical to those of FITC. We use DTAF (instead of FITC) only for conjugation with streptavidin, since fluorescence from FITC is greatly quenched after conjugation with streptavidin. This phenomenon is unique to streptavidin and is not observed with antibodies.
Cy3 is brighter, more photostable and gives less background than other orange‑red fluorescing dye conjugates. Cy3 conjugates can be excited maximally at 550 nm, with peak emission at 570 nm (Figure 2 and Table 1). For fluorescence microscopy, Cy3 can be visualised with traditional tetramethyl rhodamine (TRITC) filter sets, since the excitation and emission spectra are nearly identical to those of TRITC. We recommend Cy3 as a brighter alternative to TRITC. Cy3 can be excited to about 50% of maximum with an argon laser (514 nm or 528 nm lines), or to about 75% of maximum with a helium/neon laser (543 nm line) or mercury lamp (546 nm line). Cy3 has been used with fluorescein for double labelling; however, the use of a narrow band‑pass emission filter for fluorescein is recommended to minimise Cy3 fluorescence in the FITC filter set. Cy3 can also be paired with Alexa Fluor® 647 for multiple labelling when using a confocal microscope. However, a better choice for multiple labelling is Rhodamine Red‑X (Figure 2 and Table 1) because its fluorescence is midway between a green fluorescing dye (like Alexa Fluor® 488) and a far‑red‑fluorescing dye like Alexa Fluor® 647 (Figure 5).
RRX (Rhodamine Red X) conjugates have a peak of excitation at 570 nm and a peak of emission at 590 nm (Figure 2 and Table 1). Although TRITC has been used traditionally with FITC for double labelling, better colour separation is achieved by using RRX or Alexa Fluor® 594. Rhodamine Red‑X is particularly useful for 3‑ and 4‑colour labelling with DyLight 405, Alexa Fluor® 488 and Alexa Fluor® 647 by using a confocal microscope equipped with a 405 nm laser and a krypton/argon laser. Fluorescence from RRX lies about midway between that of Alexa Fluor® 488 and Alexa Fluor® 647 and it shows little overlap with either dye (Figure 5). The krypton‑argon laser emits lines at 488 nm, 568 nm and 647 nm, which are optimal for exciting Alexa Fluor® 488, RRX and Alexa Fluor® 647, respectively. By adding a 405 nm laser and a 420 nm emission filter, 4‑colour labelling is possible using DyLight 405‑conjugated secondary antibodies from JIR (Figure 5). The separation between all four dyes is perfect for 4‑colour labelling and all four dyes are very bright.
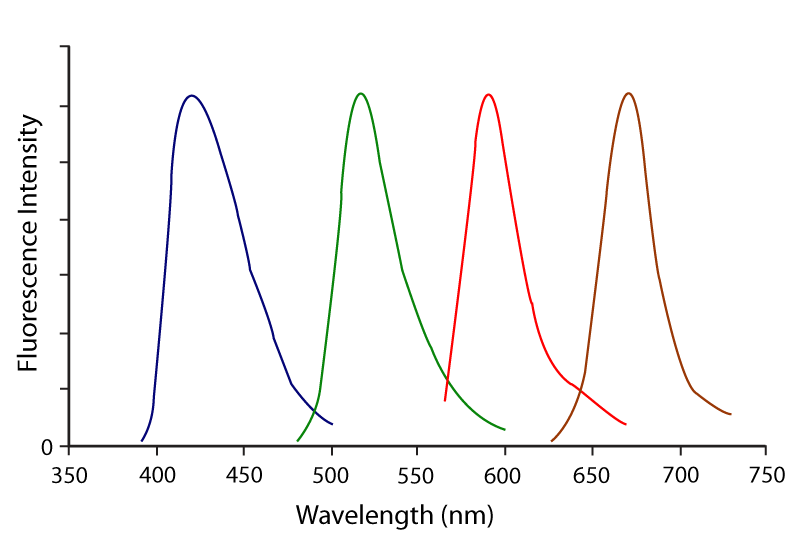
Figure 5. Emission spectra for optimal 4‑colour imaging using DyLight 405 (blue), Alexa Fluor®488 (green), Rhodamine Red‑X (red) and Alexa Fluor® 647 (purple).
Alexa Fluor® 594‑conjugated antibodies absorb light maximally around 591 nm and fluoresce with a peak around 614 nm (Figure 2 and Table 1). They are brighter, more photostable and more hydrophilic than Texas Red conjugates. Alexa Fluor® 594 conjugates are brighter than red‑fluorescing conjugates and they provide more colour separation from green‑fluorescing dyes than DyLight 549, Cy3 and TRITC conjugates. They are the best choice for immunofluorescence detection in the deep‑red region of the visible spectrum.
Alexa Fluor® 647‑conjugated antibodies absorb light maximally around 651 nm and fluoresce maximally around 667 nm (Figures 2 and 3 and Table 1). They are brighter than Cy5 and DyLight 650 in aqueous mounting media. Alexa Fluor® 647‑ and APC‑conjugated secondary antibodies are the best choice for flow cytometry when secondary antibodies fluorescing at these wavelengths are desired. Alexa Fluor® 647 conjugates are the best choice of far red‑emitting dyes for multiple‑labelling detection with a confocal microscope.
A significant advantage of using Alexa Fluor® 647 over lower wavelength‑emitting dyes is the low autofluorescence of biological specimens in this region of the spectrum. However, because of its peak emission at 667 nm, Alexa Fluor® 647 cannot be seen well by eye and it cannot be excited optimally with a mercury lamp. Therefore, Alexa Fluor® 647 is not recommended for use with conventional epifluorescent microscopes. It is most commonly visualised with a confocal microscope equipped with an appropriate laser for excitation and a far‑red detector. Alexa Fluor® 647 conjugates are less expensive alternatives to allophycocyanin conjugates for flow cytometry.
Cy5 conjugates are excited maximally at 650 nm and fluoresce maximally at 670 nm (Figure 2 and Table 1). They can be excited to about 98% of maximum with a krypton/argon laser (647 nm line) or to about 63% of maximum with a helium/neon laser (633 nm line). Cy5 can be used with a variety of other fluorophores for multiple labelling due to a wide separation of its emission from that of shorter wavelength‑emitting fluorophores. However, we recommend Alexa Fluor® 647 as the preferred far red‑fluorescing dye because it is brighter than Cy5 in aqueous mounting media. The reverse is true when mounting in non‑polar, plastic mounting media, i.e. Cy5 is brighter than other far‑red‑fluorescing dyes, including Alexa Fluor® 647 and DyLight 650. Cy5 is available conjugated to a limited selection of antibodies.
Alexa Fluor® 680 and Alexa Fluor® 790 conjugates are used for very sensitive Western blots, ELISAs and multiplexing arrays. Alexa Fluor® 680 conjugates are excited with a peak around 684 nm and fluoresce with a peak around 702 nm (Figures 2 and 3 and Table 1). Alexa Fluor® 790 conjugates are excited with a peak around 792 nm and fluoresce at a peak around 803 nm. They are the best choice for highly sensitive single or double labelling with fluorescence imaged in a LiCor Odyssey imager.
Biotin-SP (long spacer)
Biotin‑SP is our trade name for biotin with a 6 atom spacer positioned between biotin and the protein to which it is conjugated. When Biotin‑SP‑conjugated antibodies are used in enzyme immunoassays, there is an increase in sensitivity compared to biotin conjugated antibodies without the spacer. This is especially notable when Biotin‑SP conjugated antibodies are used with alkaline phosphatase‑conjugated streptavidin. Apparently, the long spacer extends the biotin moiety away from the antibody surface, making it more accessible to binding sites on streptavidin. Biotinylated antibodies require an additional reagent for visualisation. We offer streptavidin and Mouse Anti‑Biotin conjugated to fluorophores and enzymes.
Enzymes
Horseradish peroxidase (HRP) conjugates are prepared by a modified Nakane and Kawaoi procedure (J. Histochem. Cytochem. 1974. 22, 1084). Peroxidase conjugates are commonly used for immunohistochemistry, Western blotting and ELISA. Affinity purified anti‑horseradish peroxidase and conjugates are available for detection of horseradish peroxidase antigen or for signal amplification of HRP‑containing reagents. For immunostaining of mammalian cells, an advantage of using anti‑horseradish peroxidase is reduced background, because the antibody does not recognise the endogenous peroxidase‑like enzymes found in those cells.Alkaline phosphatase (from calf intestine) conjugates are prepared by a modified method of Avremeas et al., Scand. J. Immunol. 1978. 8 (Supple. 7), 7. Resulting conjugates contain heterogeneous, high molecular weight complexes. They are sensitive reagents for solid‑phase immunoassays such as ELISA and Western blotting. Although alkaline phosphatase conjugates are sometimes used for immunohistochemistry, penetration into whole mount tissues may be limited by their large sizes.