A superior strategy for validation of antibody

The combination of blocking peptide and Mass spectrometry (MS)
It is reported that the annual loss in biological and medical research caused by antibodies of inferior quality amounts to approximately 800 million U.S. dollars. Moreover, the consequences of inconsistent experimental results and poor reproducibility of antibodies are beyond estimation.
The International Antibody Validation Working Group (IWGAV) published an article entitled A Proposal for Validation of Antibodies in Nature Methods in September 2016, stressing that antibody validation should be performed in accordance with the corresponding applications and backgrounds of the targeted protein. The authors proposes five conceptual pillars for validation of antibodies: (i) genetic strategies, (ii) orthogonal strategies, (iii) independent antibody strategies, (iv) expression of tagged proteins, and (v) immune-capture followed by mass spectrometry (MS).
Each of these five methods has its own advantages and disadvantages, as well as applicable conditions. For example, genetic strategies assess antibody specificity by comparing the relevant signal in cells or tissues, in which the target gene is knocked out or knocked down using CRISPR–Cas9 or RNA interference (RNAi), with that of Wild-Type (WT) control. Although this approach is straightforward, the failure to knock down or knock out target gene will undermine the experimental feasibility of this method considerably.
The method of immune-capture followed by mass spectrometry (MS) can identify proteins which directly interact with purified antibodies, as well as additional proteins that interact indirectly with the target protein. However, this approach relies on expensive scientific instruments and experimental materials. Not every lab can afford the equipment.
Here at Affinity Biotech, the antibodies are validated using an Independent antibody validation. To that end, several antibodies are used to identify the different epitopes on the target protein. Antibody with the highest specificity is determined through a due procedure consists of comparison and quantification analysis. Although there should be no problem for the detection of most antibodies, it is still insufficient to detect antibodies with higher requirement of specificity, especially those site-specific phosphor-antibodies.
By incorporating years of experience in antibody development and production, Affinity Biotech adopts a more appropriate strategy for antibody verification: The combination of blocking peptide and Mass spectrometry (MS).
The blocking peptide (antigen) and the antibody are of one-to-one correspondence. . This is the most primitive material that only the original manufacturers can provide.
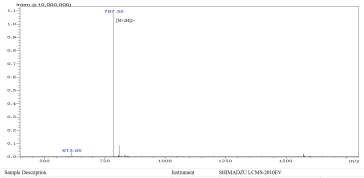
For starters, we perform a MS analysis of the blocking peptide to ensure that the peptide sequence is accurate.
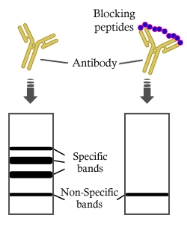
In Western Blotting assay, the peptide blocks the signal of the target antibody. A lane without blocking peptide is used as control. The blocking peptide will compete against the target protein for binding site, leaving no specific bands in the blocking group.
This strategy is currently used in the validation of our antibodies. It is vital for the production of phosphor-antibodies, because it ensures the site-specificity of phosphor-antibodies. In other words, the phosphorylated antibodies can and will only recognize the phosphorylated target protein at the corresponding site. Non-phosphorylated target protein or target protein phosphorylated at other site will not be recognized.
Let us make an example. In the Western Blotting validation of Phospho-MSK1 (Ser211) Antibody (Cat. No. AF7155), non-phosphorylated blocking peptide and phosphorylated blocking peptide are added in Lanes 1 and Lane 3, respectively. Lane 2 is not blocked. The results show that after blocking the target protein using phosphorylated peptide, no band of interest is detected.

If you have any questions about our blocking peptides, please feel free to contact us. With confidence, Affinity Biotech will provide corresponding blocking peptide for each and every antibody. The customers are welcome to perform antibody validation using these blocking peptides by yourself.
At this juncture, you may doubt since the combination of blocking peptide and Mass spectrometry (MS) is superior in antibody validation, is there any other company in the world uses the same strategy? The answer is positive!
Alomone Labs Ltd in Israel, a leading supplier of Ion Channel and signaling research tools, has harvested more than 13,000 references across the world with 700 antibody products. Alomone Labs uses the combination of blocking peptide and Mass spectrometry in antibody validation. This is sufficient to demonstrate the reliability of this validation approach.