IL-8 Leeporter™ Luciferase Reporter-RAW264.7 Cell Line
Catalogue Number: 14-101ACL-ABO
| Manufacturer: | Abeomics |
| Type: | Cell Lines |
| Shipping Condition: | Dry Ice |
| Storage Condition: | Liquid N2 |
| Unit(s): | 1 vial |
| Application: | FA |
Description
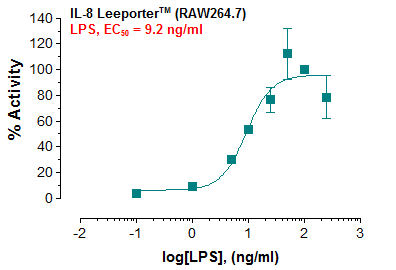
Description: The IL-8 Leeporter™ Luciferase Reporter cell line is a stably transfected RAW 264.7 cell line which expresses Renilla luciferase reporter gene under the transcriptional control of the IL-8 promoter. IL-8 is one of the key proinflammatory chemokines or cytokines, which is produced by macrophages and other epithelial cells. Induction of IL-8 is associated with inflammation. The IL-8 induction by Toll-like receptor 4 (TLR4) ligand, LPS, is shown in Figure 1.
Additional Text
Application Notes
Application: Monitor the IL-8 induction activity. Screen for activators or inhibitors of the IL-8 induction. Culture conditions: Cells should be grown at 37°C with 5% CO2 using DMEM medium (w/ L-Glutamine, 4.5g/L Glucose and Sodium Pyruvate) supplemented with 10% heat-inactivated FBS and 1% Pen/Strep, plus 3 µg/ml of Puromycin (Note: Puromycin can be omitted during the reporter cell assays). It is recommended to quickly thaw the frozen cells upon receipt or from liquid nitrogen in a 37°C water-bath, transfer to a tube containing 10 ml of growth medium without Puromycin, spin down cells, resuspend cells in pre-warmed growth medium without Puromycin, transfer resuspended cells to T25 flask and culture in 37°C-CO2 incubator. Leave the T25 flask in the incubator for 1~2 days without disturbing or changing the medium until cells completely recover viability and become adherent. Once cells are over 90% adherent, remove growth medium and passage the cells through trypsinization and centrifugation. At first passage, switch to growth medium containing Puromycin. Cells should be split before they reach complete confluence. Note: RAW264.7 cells may not be detached well by trypsinization only. So you may need to use a cell scraper to harvest the trypsinized cells. To passage the cells, detach cells from culture vessel with Trypsin/EDTA, add complete growth medium and transfer to a tube, spin down cells, resuspend cells and seed appropriate aliquots of cells suspension into new culture vessels. Subcultivation ration = 1:10 to 1:20 weekly. To achieve satisfactory results, cells should not be passaged over 16 times. Functional validation: A. Response of IL-8 Leeporter™ - RAW264.7 cells to lipopolysaccharide (LPS) 1. Plate IL-8 Leeporter™ - RAW264.7 cells into a white solid-bottom 96-well microplate in 100 µl of growth medium at 1 x 105 cells/well and incubate cells at 37°C in a CO2 incubator for 4-6 hours. 2. Stimulate cells with different concentrations of LPS and incubate cells at 37°C in a CO2 incubator for 16 hours. 3. Equilibrate the plate to room temperature for 10 minutes. 4. Add 50 µl of luciferase assay reagent (Abeomics, Cat #17-1101; Refer to the reagent datasheet for the detailed luciferase assay protocol) per well. 5. Read the plate in 1-5 minutes to measure luminescence using a microplate luminometer.
Storage Note
Immediately upon receipt, store in liquid nitrogen.