NFAT Leeporter™ Luciferase Reporter-RAW264.7 Cell Line
Catalogue Number: 14-111ACL-ABO
| Manufacturer: | Abeomics |
| Type: | Cell Lines |
| Shipping Condition: | Dry Ice |
| Storage Condition: | Liquid N2 |
| Unit(s): | 1 vial |
| Application: | FA |
Description
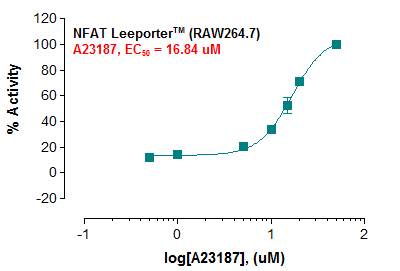
Description: The NFAT Leeporter™ Luciferase Reporter cell line is a stably transfected RAW264.7 cell line which expresses Renilla luciferase reporter gene under the transcriptional control of the Nuclear Factor of Activated T-cells (NFAT) response element, so that the cell line is designed to measure the transcriptional activity of NFAT. NFAT is a transcription factor originally found in activated T lymphocytes, and is now known to regulate not only T cell activation and differentiation but also the function of other immune cells including dendritic cells, B cells and megakaryocytes. The NFAT induction by calcium ionophore A23187 is shown in Figure 1.
Additional Text
Application Notes
Application: Monitor the NFAT signaling pathway activity. Screen for activators or inhibitors of the NFAT signaling pathway. Culture conditions: Cells should be grown at 37°C with 5% CO2 using DMEM medium (w/ L-Glutamine, 4.5g/L Glucose and Sodium Pyruvate) supplemented with 10% heat-inactivated FBS and 1% Pen/Strep, plus 3 µg/ml of Puromycin (Note: Puromycin can be omitted during the reporter cell assays). It is recommended to quickly thaw the frozen cells upon receipt or from liquid nitrogen in a 37°C water-bath, transfer to a tube containing 10 ml of growth medium without Puromycin, spin down cells, resuspend cells in pre-warmed growth medium without Puromycin, transfer resuspended cells to T25 flask and culture in 37°C-CO2 incubator. Leave the T25 flask in the incubator for 1~2 days without disturbing or changing the medium until cells completely recover viability and become adherent. Once cells are over 90% adherent, remove growth medium and passage the cells through trypsinization and centrifugation. At first passage, switch to growth medium containing Puromycin. Cells should be split before they reach complete confluence. Note: RAW264.7 cells may not be detached well by trypsinization only. So you may need to use a cell scraper to harvest the trypsinized cells. To passage the cells, detach cells from culture vessel with Trypsin/EDTA, add complete growth medium and transfer to a tube, spin down cells, resuspend cells and seed appropriate aliquots of cells suspension into new culture vessels. Subcultivation ration = 1:10 to 1:20 weekly. To achieve satisfactory results, cells should not be passaged over 16 times. Functional validation: A. Response of NFAT Leeporter™ - RAW264.7 cells to calcium ionophore A23187. 1. Plate NFAT Leeporter™ - RAW264.7 cells into a white solid-bottom 96-well microplate in 100 ul of growth medium at 1 x 105 cells/well and incubate cells at 37°C in a CO2 incubator for 4-6 hours. 2. Stimulate cells with different concentrations of calcium ionophore A23187 and incubate cells at 37°C in a CO2 incubator for 16 hours. , 3. Equilibrate the plate to room temperature for 10 minutes. 4. Add 50 µl of luciferase assay reagent (Abeomics, Cat #17-1101; Refer to the reagent datasheet for the detailed luciferase assay protocol) per well. 5. Read the plate in 1-5 minutes to measure luminescence using a microplate luminometer.
Storage Note
Immediately upon receipt, store in liquid nitrogen.