Nrf2 Leeporter™ Luciferase Reporter-MCF7 Cell Line
Catalogue Number: 14-117ACL-ABO
| Manufacturer: | Abeomics |
| Type: | Cell Lines |
| Shipping Condition: | Dry Ice |
| Storage Condition: | Liquid N2 |
| Unit(s): | 1 vial |
| Application: | FA |
Description
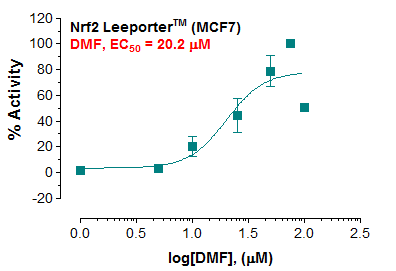
Description: The Nrf2 Leeporter™ Luciferase Reporter cell line is a stably transfected MCF7 cell line which expresses Renilla luciferase reporter gene under the transcriptional control of the antioxidant response element (ARE). ARE is known to regulate expression and induction of various detoxifying enzyme genes in response to antioxidants and xenobiotics, and is primarily regulated by the Keap1-Nrf2 pathway in which induction and nuclear translocation of Nrf2 mediated by antioxidants and xenobiotics results in the binding of Nrf2 to ARE leading to the expression of defensive genes. The Nrf2 induction by dimethyl fumarate (DMF) is shown in Figure 1.
Additional Text
Application Notes
Application: Monitor the Nrf2 induction activity. Screen for activators or inhibitors of the Nrf2 signaling pathway. Culture conditions: Cells should be grown at 37°C with 5% CO2 using Advanced Minimum Essential Medium (Advanced MEM; e.g. Gibco #12491) supplemented with 10% heat-inactivated FBS, 2 mM glutamine and 1% Pen/Strep, plus 2 µg/ml of Puromycin (Note: Puromycin can be omitted during the reporter cell assays). It is recommended to quickly thaw the frozen cells upon receipt or from liquid nitrogen in a 37°C water-bath, transfer to a tube containing 10 ml of growth medium without Puromycin, spin down cells, resuspend cells in pre-warmed growth medium without Puromycin, transfer resuspended cells to T25 flask and culture in 37°C-CO2 incubator. Leave the T25 flask in the incubator for 2~4 days without disturbing or changing the medium until cells completely recover viability and become adherent. Once cells are over 90% adherent, remove growth medium and passage the cells through trypsinization and centrifugation. At first passage, switch to growth medium containing Puromycin. Cells should be split before they reach complete confluence. To passage the cells, detach cells from culture vessel with Trypsin/EDTA, add complete growth medium and transfer to a tube, spin down cells, resuspend cells and seed appropriate aliquots of cells suspension into new culture vessels. Subcultivation ration = 1:10 to 1:20 weekly. To achieve satisfactory results, cells should not be passaged over 16 times. Functional validation: A. Response of Nrf2 Leeporter™ - MCF7 cells to dimethyl fumarate (DMF). 1. Harvest Nrf2 Leeporter™ - MCF7 cells and seed cells into a white solid-bottom 96-well microplate in 100 µl of growth medium at 5 x 10^4 cells/well. 2. Incubate cells at 37°C in a CO2 incubator for overnight. 3. The next day, stimulate cells with various concentrations of DMF. 4. Incubate at 37°C in a CO2 incubator for 16 hours. 5. Equilibrate the plate to room temperature for 10 minutes. 6. Add 50 µl of luciferase assay reagent (Abeomics, Cat #17-1101; Refer to the reagent datasheet for the detailed luciferase assay protocol) per well. 7. Read the plate in 1-5 minutes to measure luminescence using a microplate luminometer.
Storage Note
Immediately upon receipt, store in liquid nitrogen.