STAT3 Leeporter™ Luciferase Reporter-NIH 3T3 Cell Line
Catalogue Number: 14-149ACL-ABO
| Manufacturer: | Abeomics |
| Type: | Cell Lines |
| Shipping Condition: | Dry Ice |
| Storage Condition: | Liquid N2 |
| Unit(s): | 1 vial |
| Application: | FA |
Description
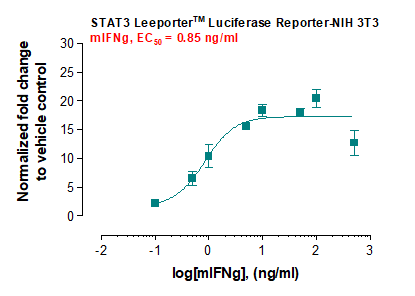
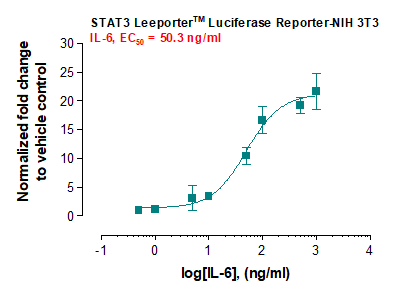
Description: The STAT3 Leeporter™ Luciferase Reporter cell line is a stably transfected NIH 3T3 cell line which expresses Renilla luciferase reporter gene under the transcriptional control of the STAT3 responsive promoter, so that the cell line is designed to measure the transcriptional activity of STAT3. As a transcription factor, Signal Transducer and Activator of Transcription 3 (STAT3) is activated through phosphorylation at tyrosine 705 in response to various cytokines. The STAT3 induction by interferon gamma and IL-6 is shown in Figures 1 and 2.
Additional Text
Application Notes
Application: Monitor the STAT3 signaling pathway activity. Screen for activators or inhibitors of the STAT3 signaling pathway. Culture conditions: Cells should be grown at 37°C with 5% CO2 using DMEM medium (w/ L-Glutamine, 4.5g/L Glucose and Sodium Pyruvate) supplemented with 10% heat-inactivated FBS and 1% Pen/Strep, plus 3 µg/ml of Puromycin (Note: Puromycin can be omitted during the reporter cell assays). It is recommended to quickly thaw the frozen cells upon receipt or from liquid nitrogen in a 37°C water-bath, transfer to a tube containing 10 ml of growth medium without Puromycin, spin down cells, resuspend cells in pre-warmed growth medium without Puromycin, transfer resuspended cells to T25 flask and culture in 37°C-CO2 incubator. Leave the T25 flask in the incubator for 1~3 days without disturbing or changing the medium until cells completely recover viability and become adherent. Once cells are between 80~90% adherent, remove growth medium and passage the cells through trypsinization and centrifugation. At first passage, switch to growth medium containing Puromycin. Note: NIH 3T3 cells should be split before they reach 90% confluence; otherwise, they become self-lifted and aggregate irrevisibly. Precoating the cell plates with 0.1% gelatin may prevent NIH 3T3 cells from self-lifting. During cell trypsinization, cells covered enough with trypsin-EDTA solution should be stayed at 37°C for 10 min without agitation. To passage the cells, detach cells from culture vessel with Trypsin/EDTA, add complete growth medium and transfer to a tube, spin down cells, resuspend cells and seed appropriate aliquots of cells suspension into new culture vessels. Subcultivation ration = 1:10 to 1:20 weekly. To achieve satisfactory results, cells should not be passaged over 16 times. Functional validation: A. Response of STAT3 Leeporter™ - NIH 3T3 cells to mIFN-gamma. 1. Plate STAT3 Leeporter™ - NIH 3T3 cells into a white solid-bottom 96-well microplate in 100 µl of growth medium at 1 x 10^5 cells/well and incubate the plate at 37°C in a CO2 incubator for 2-3 hours. 2. Stimulate cells with various concentrations of mIFN-gamma and incubate cells at 37°C in a CO2 incubator for 16 hours. 3. Equilibrate the plate to room temperature for 10 minutes. 4. Add 50 µl of luciferase assay reagent (Abeomics, Cat #17-1101; Refer to the reagent datasheet for the detailed luciferase assay prot°Col) per well. 5. Read the plate in 1-5 minutes to measure luminescence using a microplate luminometer. A. Response of STAT3 Leeporter™ - NIH 3T3 cells to IL-6. 1. Plate STAT3 Leeporter™ - NIH 3T3 cells into a white solid-bottom 96-well microplate in 100 µl of growth medium at 1 x 10^5 cells/well and incubate the plate at 37°C in a CO2 incubator for 2-3 hours. 2. Stimulate cells with various concentrations of IL-6 and incubate cells at 37°C in a CO2 incubator for 16 hours. 3. Equilibrate the plate to room temperature for 10 minutes. 4. Add 50 µl of luciferase assay reagent (Abeomics,
Storage Note
Immediately upon receipt, store in liquid nitrogen.