Quanta BioDesign, Ltd. is proud to announce that our newest click chemistry crosslinker, Aminooxy-dPEG®11-azide∙HCl, product number 10538, is available for sale. This crosslinker combines carboxyl reactivity via an aminooxy group and alkyne reactivity through an azide group. See Figure 1 for the structure.
Aminooxy-dPEG®11-azide: A New Click Chemistry Crosslinker
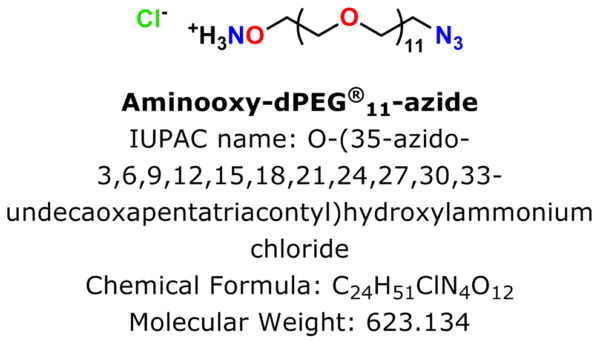
Figure 1: Aminooxy-dPEG®11-azide·HCl, product number 10538, is Quanta BioDesign’s newest click chemistry crosslinker.
An azide group sits at one end of a 38 atom (40.8 Å) discrete polyethylene glycol (dPEG®) chain, while an aminooxy group (as the HCl salt) sits at the other end. In this crosslinker, the azide group permits many types of click chemistry reactions, including copper-catalyzed (CuAAC), ruthenium-catalyzed (RuAAC), and copper-free (also called “strain promoted,” and known by the acronym SPAAC) click chemistry. Also, the aminooxy group reacts with aldehydes and ketones to form oxime bonds. The bond is named an aldoxime if the reaction occurs between an aldehyde and an aminooxy. If the bond forms between a ketone and an aminooxy, the bond is called a ketoxime. Figure 2 shows the general scheme for CuAAC and SPAAC reactions, while Figure 3 shows the reactions that form aldoximes and ketoximes, plus the catalysts that enable bond formation.
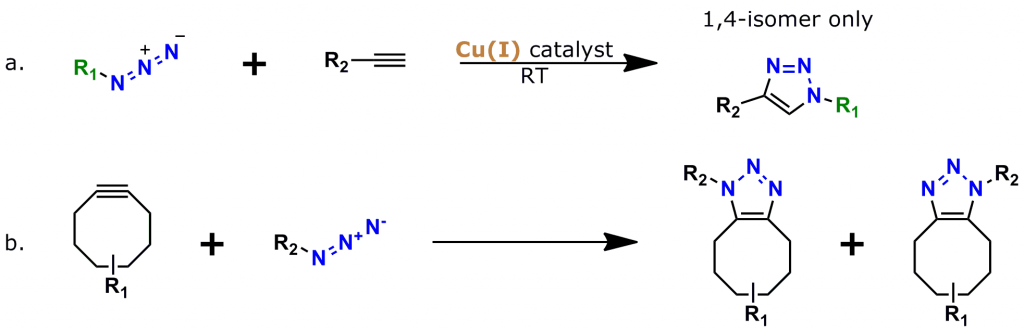
Figure 2: The two most popular types of click chemistry. a. Copper-catalyzed azide-alkyne cycloaddition (CuAAC), the “classic” click chemistry reaction that was discovered in 2002. b. Strain-promoted azide-alkyne cycloaddition (SPAAC, also known as “copper-free click chemistry”), discovered in 2005-2006 by Carolyn Bertozzi and colleagues.
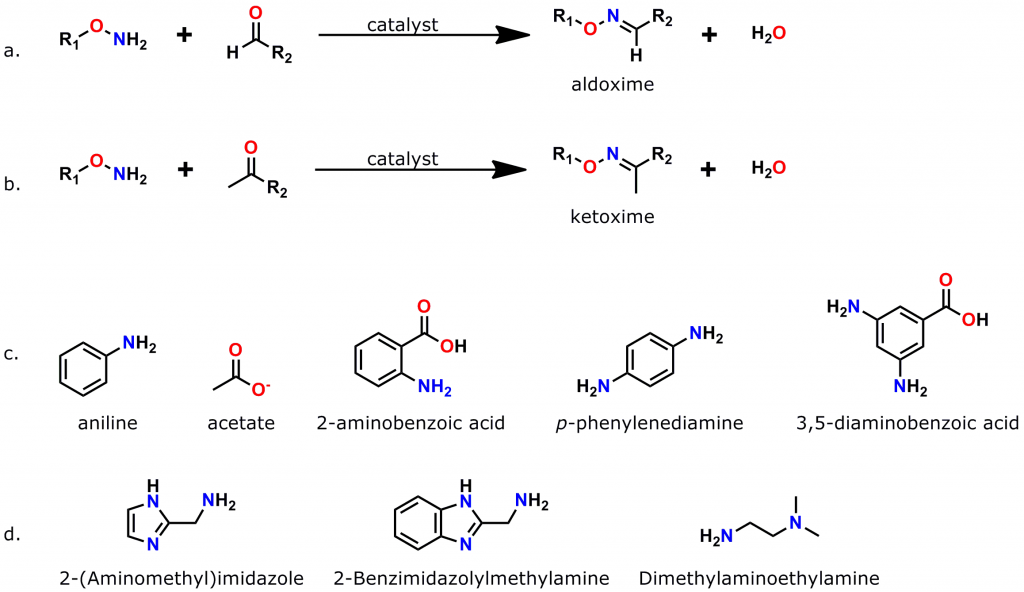
Figure 3: Oxime bond formation is a specific, bioorthogonal reaction that can be greatly accelerated by the use of suitable catalysts. a. Scheme for the formation of aldoximes, when an aminooxy compound reacts with an aldehyde. b. Scheme for the formation of a ketoxime (ketone plus aminooxy group). c. Common catalysts used to accelerate oxime bond formation. d. Recently discovered low-toxicity catalysts for oxime bond formation.
The Click Chemistry End of the Molecule
Click chemistry is a commonly used means of constructing new, complex molecules rapidly. Click chemistry applications include drug development, antibody-drug conjugates, nanotechnology, macromolecular construction, and dendrimer synthesis, among many, wide-ranging areas of chemical synthesis.
The Aminooxy Terminus
Oximes have been studied since 1882. Today, they find widespread use in bioconjugation reactions. In 2017, Kölmel and Kool published a detailed review of the mechanisms and catalysis of oxime and hydrazone bond formation in bioconjugation reactions. [1] I highly recommend this review.
What Makes Aminooxy-dPEG®11-azide∙HCl a Great Product?
Of course, a great crosslinker isn’t all that great if it can’t be used to solve problems. What can Aminooxy-dPEG®11-azide∙HCl do that makes it so great?
First, our dPEG® products are amphiphilic, which means that they are soluble in both water and many organic solvents. Thus, syntheses with Aminooxy-dPEG®11-azide∙HCl run in water, organic solvents, or both. This flexibility in the choice of solvents allows the user to work with biomolecules in biocompatible aqueous buffers or with aqueous buffers plus small amounts of water-miscible organic solvents. Notably, both click chemistry and oxime bond formation are compatible with water.
Second, the azide and aminooxy groups are both orthogonal to each other and bioorthogonal.[2] Although ketones and aldehydes do occur in living cells, the natural occurrence of these reactive groups is quite small. Usually, when you want to react aldehydes or ketones in vivo or in vitro with a suitable reaction partner, you first create the ketones or aldehydes on the carbohydrate coats of glycoproteins using sodium periodate. For click chemistry reactions, the azide-alkyne reaction pairs are almost entirely unknown and unavailable in living organisms unless they have been introduced through synthetic biology or chemistry methods. Thus, unlike many types of reactions that bioconjugation scientists carry out in living cells, neither end of this molecule is likely to react inappropriately.
Third, a similar product with a much shorter PEG linker has already been used in a patented process developed by Peiming Zhang and Lindsay Stuart, both of whom are at the BioDesign Institute at Arizona State University.[3] In Zhang’s and Stuart’s patent, a PEG5 linker functionalized with azide and aminooxy groups was used to attach a ketone-functionalized oligosaccharide to a bead-bound DNA molecule that was conjugated to the SPAAC cyclooctyne bicyclo[6.1.0]nonyne, or BCN. A DNA polymerase then regulated the movement of the oligosaccharide through a nanopore at a controlled speed. Then, by using mass spectrometry, each residue of the oligosaccharide could be detected and identified in its proper sequence order.
What Can You Do with Aminooxy-dPEG®11-azide∙HCl?
So, what can you do with Aminooxy-dPEG®11-azide∙HCl? Frankly, that’s up to you. There are endless possible applications for this product! Most uses will require the functionalization of oligosaccharides or polysaccharides with aldehydes or ketones via sodium periodate. Glycomics applications may benefit from this product.
Oximes often are used to create prodrugs that activate in vivo. Thus, drug development and drug delivery may benefit from the use of Aminooxy-dPEG®11-azide∙HCl. For example, oligosaccharides exert protective, stimulatory, and inhibitory effects on cells in vivo and are part of cell recognition. It may be possible to use Aminooxy-dPEG®11-azide∙HCl to couple oligosaccharides with defined effects on cells to small molecule compounds that modulate other activities or effects within cells.
However, the choices of applications are really up to the user.
Aminooxy-dPEG®11-azide∙HCl is Scalable
Our standard package sizes for Aminooxy-dPEG®11-azide∙HCl, product number 10538, are 50 and 1000 mg. Our commercial capabilities permit us to manufacture this product at any scale that you need.
So, please, navigate over to the product page for Aminooxy-dPEG®11-azide∙HCl, and click the “Add to Cart” button. You can discover the dPEG® difference for yourself with our newest click chemistry crosslinker. Go ahead! What are you waiting for? Do it now.
References
[1] Kölmel, D. K.; Kool, E. T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117(15), 10358–10376. https://doi.org/10.1021/acs.chemrev.7b00090.
[2] For readers fascinated by the etymology of words, the following may be of interest. The roots of this word come from ancient Greek. “Bio” means “life,” and the word “orthogonal” (from orthogonios) means “right-angled” or, by analogy, “unrelated.” Thus, a bioorthogonal chemical reaction is one that is unrelated to processes that occur normally in living systems.
[3] Zhang, P.; Lindsay, S. Translocation of a Non-Nucleic Acid Polymer Using a Polymerase. WO2018064078A1, April 5, 2018. https://patents.google.com/patent/WO2018064078A1/en