Quanta BioDesign’s monodispersed, discrete PEG (dPEG®) products include a wide range of useful tools for click chemistry. Most of our reagents are designed to enable copper-free click chemistry, which is essential for biocompatible labeling and crosslinking. Copper-free click chemistry that allows crosslinking of an amine to an azide is the subject of this post.
The Discovery of Copper-Free Click Chemistry
This post provides a short synopsis of the discovery of copper-free click chemistry. For a comprehensive review of click chemistry and its applications with dPEG® compounds.
Copper-free click chemistry is an indispensable tool in the toolbox of biotechnologists worldwide. Carolyn Bertozzi and coworkers discovered copper-free click chemistry (formally known as strain-promoted azide-alkyne cycloaddition, or SPAAC) in 2004. Bertozzi was looking for bioconjugation techniques that were both bioorthogonal and biocompatible (1). The copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction independently discovered in 2002 by Meldal’s group (2) and Sharpless and coworkers (3) uses copper(I) salts that are often toxic to live cells. The cytotoxicity of these copper salts spurred the development of less toxic and non-toxic alternatives that are biocompatible.
Bertozzi developed SPAAC after reading a 1961 paper by Wittig and Krebs that reported that phenyl azide and cyclooctyne react violently (4). From that clue, the Bertozzi lab developed and tested cyclooctyne derivatives to find a compound with optimal stability, water solubility, and reactivity with azides. Other labs joined the search, and today several different strained cyclooctyne compounds find use in copper-free click chemistry reactions.
Quanta BioDesign’s Copper-Free Click Chemistry Products for Crosslinking Amines with Azides
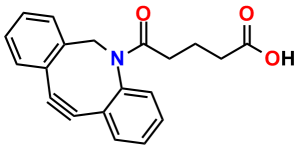
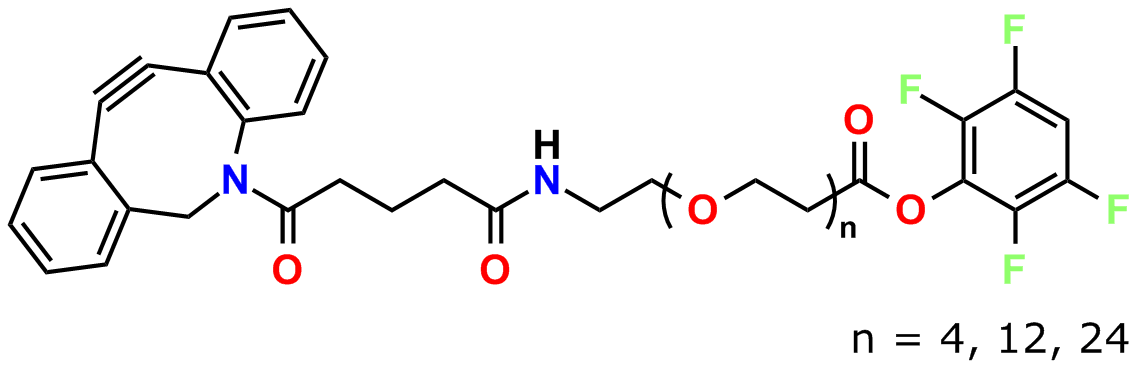
To conjugate a dPEG® crossbridge to an azide via copper-free click chemistry, Quanta BioDesign chose the strained cyclooctyne known as DBCO (for dibenzylcyclooctyne; also known as DIBAC, or ADIBO) See Figure 1. Researchers in the laboratory of Floris van Delft at Radboud University Nijmegen, The Netherlands, developed DBCO (5). The compound has broad solubility, high reactivity with azides, and excellent stability. To DBCO, Quanta BioDesign conjugates various lengths of amino-dPEG®-acid spacers to create a water-soluble, amine-reactive, and copper-free click chemistry reagent. We then functionalize the acid with 2,3,5,6-tetrafluorophenol (TFP), forming the active TFP ester for amine reactivity.