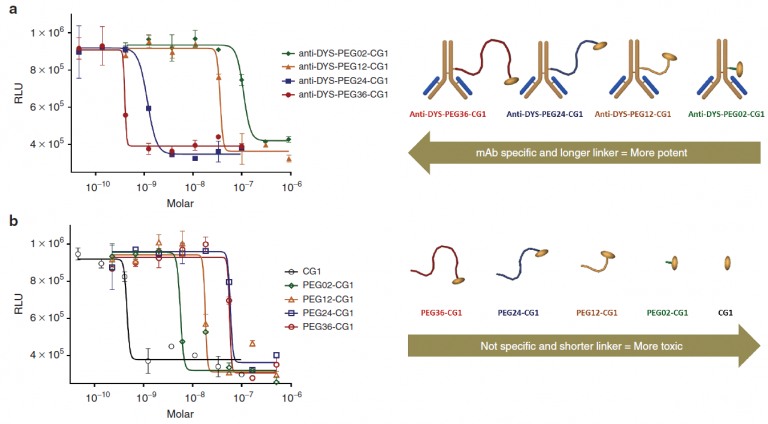
Pharmaceutical company Centrose, founded by James R. Prudent, Ph.D., developed a new class of antibody drug conjugates called extracellular drug conjugates. Nature Publishing Group published the research as a open access paper in its Molecular Therapy journal.1 Apart from the interesting and important development of a new class of antibody drug conjugate (ADC), the research also showed how important linker length 2 is to the potency and specificity of the EDC.
How Extracellular Drug Conjugates Work
Extracellular drug conjugates (EDCs) are designed similarly to ADCs. That is, EDCs consist of a monoclonal antibody (mAb), a linker, and a cytotoxic agent. Antibody drug conjugates require internalization into a diseased cell where the cytotoxic agent can then be released to act on its target.3 The cytotoxic agent may require intracellular modification or degradation to act on its target molecule.
By contrast, extracellular drug conjugates require no internalization. Rather, EDCs target cell surface proteins that are expressed on a target (i.e., cancerous) cell. Moreover, the cytotoxic agent that is linked to the mAb does not target the same protein targeted by the mAb. Rather, the cytotoxic agent kills the targeted cells by affecting a protein or enzyme that is different from the protein or enzyme bound by the mAb but that is closely associated with the target protein or enzyme.
In this particular study, the research team observed that some proteins that are overexpressed on the surface of cancer cells are closely associated with the sodium potassium ATPase1 (NKA, and also known as the sodium potassium pump; see Figure 1). Cardiac glycosides such as digoxin or ouabain inhibit the NKA. Cells affected by these or other cardiac glycosides swell, then undergo necrotic cell death.1,4,5 The team reasoned that by (1) combining antibodies to proteins that are both (a) overexpressed on the cell surface of cancer cells and (b) closely associated with the NKA (2) with cardiac glycosides that strongly inhibit the NKA (3) would create cancer therapeutic antibody drug conjugates that were localized to the extracellular space, hence, Extracellular Drug Conjugates.
Creating the Extracellular Drug Conjugates in this Study
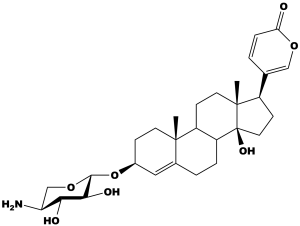
Through prior testing (not in this paper) the team found that the cardiac glycoside scillarenin β-L-aminoxyloside (Figure 1) highly inhibited the NKA. This was chosen as the drug for conjugation to the antibody.