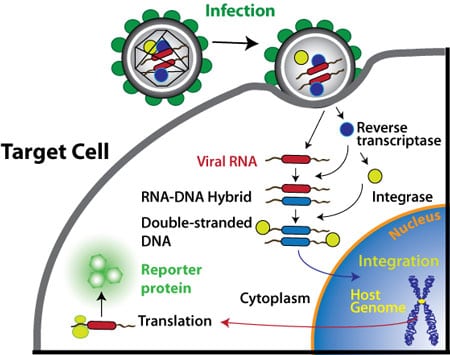
How Lentivirus Delivery Works
The pseudoviral particles can infect target cells and express genes, shRNAs, microRNAs and reporter molecules but cannot replicate within the target cells for the following two reasons:
1) The viral structure genes are absent
2) The Long Terminal Repeats (LTRs) are designed to be self-inactivating upon infection and integration
Following successful infection and integration (also termed “transduction”) into the target cells, the expression cassette is continuously and stably produces high levels of effector or reporter molecules in the transduced cells. target cells stably expressing the engineered molecule or gene can be isolated using the selectable marker contained in the expression lentivector construct (e.g., puromycin, neomycin, copGFP, RFP, etc.)
Frequently Asked Lentivirus Questions (FAQs)
|
Q:
|
What are lentiviruses? | ||||||||||||||||
|
A:
|
Lentiviruses are a type of retrovirus that can be used to deliver cDNA, shRNAs, microRNAs, or transcription reporters to dividing and non-dividing cells. Once introduced into the target cells, the introduced transgene integrates into the host cell genome to provide permanent expression of the transgene.
|
||||||||||||||||
|
Q:
|
What are the relative elements and percentages of the HIV viral genome present in SBI’s lentivectors? | ||||||||||||||||
|
A:
|
There is about 20% HIV sequence in SBI’s lentivectors. Below are examples of the percentage of the HIV genome found in CD510B-1 (pCDH-CMV-MCS-EF1-Puro) (7377 bps)
|
||||||||||||||||
|
Q:
|
Will SBI’s VSV-G pseudotyped lentivirus particles infect my cells of interest? | ||||||||||||||||
|
A:
|
Most cell-types and tissues tested are capable of successful lentiviral transduction. To view a profile of cell lines evaluated, download this PDF.LentiDelivery (PDF).
|
||||||||||||||||
|
Q:
|
What are the Biosafety concerns when handling SBI’s lentiviruses? | ||||||||||||||||
|
A:
|
The basic requirements are a working laminar flow hood and biosafety level 2 procedures. Follow this link to view the recommendations by the Center for Disease Control (CDC website information)
|
||||||||||||||||
|
Q:
|
What is the size of insert which can be cloned into the vector? And why? | ||||||||||||||||
|
A:
|
This depends upon the lentivector you are choosing. The region between the 5’and 3’LTR must not exceed 9kb, which is the size of the native HIV genome. To calculate the size of the insert that the lentivector can accommodate, download the vector sequence and determine the size of this region. The difference between 9kb and the size of the 5’- to 3’-LTR is the limit of the size of the insert. Typically most SBI lentivectors can accommodate a fragment of 4 kb, however the all-in-one SparQ vectors can only accommodate up to 3 kb. The reasons for the limitation are there is only so much room in the virus capsid, integration efficiency also goes down, and transcription slows down because of the large size.
|
||||||||||||||||
|
Q:
|
Can I make a stable cell line that will continuously produce lentiviral particles? | ||||||||||||||||
|
A:
|
No. For biosafety reasons it is not possible to create a stable cell line to continuously produce lentiviral particles. For a full protocol on how to produce lentiviral particles, please see: Lentiviral Expression Systems user manual.
|
||||||||||||||||
|
Q:
|
What are the excitation and emission spectra for SBI’s fluorescent protein markers? | ||||||||||||||||
|
A:
|
For the copGFP and dscGFP markers, those data can be viewed here GFP PDF. The Red Fluorescent Protein specifications can be found in this document RFP PDF. |