Background
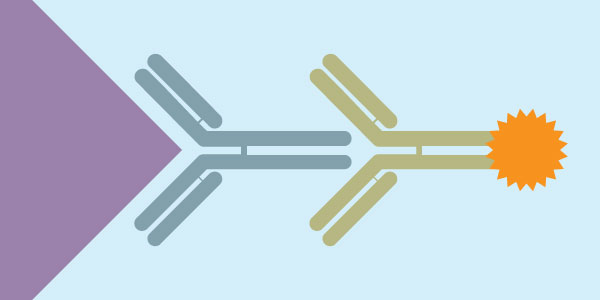
Immunocytochemistry is an immunological technique that is very similar to Immunohistochemistry. Immunocytochemistry is used to visualize the presence of a specific protein or antigen in cells (cultured cells, cell suspensions), rather than tissues. The types of cell samples that can be investigated include blood smears, cultured cells, cell suspensions, and cytospins. Each type of cell sample is prepared and treated slightly differently, but the fundamental use of primary antibody, secondary antibody and color development is very similar between Immunocytochemistry and Immunohistochemistry.
For immunocytochemistry, sample preparation involves fixing the target cells to a slide. Cells can be attached to a solid surface by several methods: adherent cells may be grown on microscope slides; cell suspensions can be centrifuged onto glass slides (cytospin),or bound to solid support using chemical linkers.
To ensure access of the antibody to its antigen, cells must be fixed and permeabilized. In an ideal situation, fixation would immobilize the antigens while retaining native cellular architecture and permitting unhindered access of antibodies to all cells and subcellular compartments.
Fixation methods fall generally into two groups: organic solvents and cross-linking reagents. Organic solvents, such as alcohols and acetone, remove lipids and dehydrate the cells, while precipitating the proteins on the cellular architecture. Cross-linking reagents (such as paraformaldehyde) form intermolecular bridges, normally through free amino groups, thus creating a network of linked antigens. Cross-linkers preserve cell structure better than organic solvents, but may alter the structure of some cell components, so much so that they are not recognized by the primary antibody.
Fixation Methods
- Acetone Fixation Fix cells in -20°C acetone for 5-10 minutes. No permeabilization step is needed following acetone fixation.
- Methanol Fixation Fix cells in -20°C methanol for 5-10 minutes. No permeabilization step needed following methanol fixation.
- Ethanol Fixation Fix cells in cooled 95% ethanol, 5% glacial acetic acid for 5-10 minutes.
- Methanol-Acetone Fixation Fix cells in cooled methanol, 10 minutes at -20°C. Remove excess methanol. Permeabilize with cooled acetone for 1 minute at –20°C.
- Methanol-Acetone Fixation Prepare 1:1 methanol and acetone mixture and fix cells at -20°C for 5-10 minutes.
- Methanol-Ethanol Fixation Prepare 1:1 methanol and ethanol mixture and fix cells at -20°C for 5-10 minutes.
- Formalin Fixation Fix cells in 10% neutral buffered formalin for 5-10 minutes. Rinse briefly with PBS. Permeabilize with 0.5% Triton X-100 for 10 minutes.
- Paraformaldehyde-Triton Fixation Fix cells in 3-4% paraformaldehyde for 10-20 minutes. Rinse briefly with PBS. Permeabilize with 0.5% Triton X-100 for 10 minutes.
- Paraformaldehyde-Methanol Fixation Fix cells in 4% paraformaldehyde for 10-20 minutes. Rinse briefly with PBS. Permeabilize with cooled methanol for 5-10 minutes at -20°C.
Permeabilization Methods
Cross-linkers typically require the addition of a permeabilization step to allow access of the antibody to the specimen. Two groups of compounds can be used for permeabilization: organic solvents and mild surfactants. Alcohols and acetone can be used as a one step fixation/permeabilization method. In addition to their fixative behavior which precipitates proteins in place, these solvents also dissolve membrane lipids which render the membrane permeable to antibodies. In the surfactant category, saponin, Triton X-100, Tween-20, etc, are used for their ability to disrupt membranes.
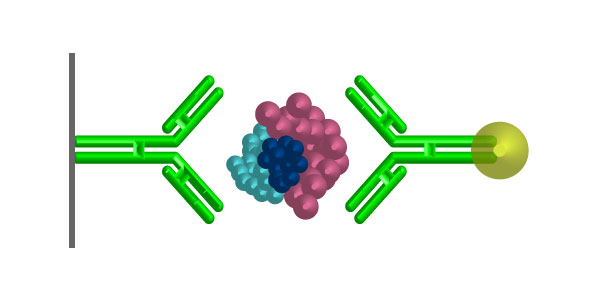
Immunocytochemistry (HRP detection)
Materials Required
- Deionized water
- PBS
- Triton X-100
- Hydrogen Peroxide (H2O2)
- Primary Antibody
- Biotinylated secondary antibody (or HRP-conjugated secondary antibody)
- Bovine Serum Albumin (BSA)
- Streptavidin-HRP
- DAB
- Glycerol
- Hematoxylin (optional)
- Acetic Acid (optional)
Sample Preparation
- Transfer cell culture to the wells of a chamber slide or slides of your choice. Allow cells to grow to confluence with the addition of fresh media.
- Wash the cells thoroughly 5×2 min in PBS.
Fixation Step
- Fix cells with appropriate fixation method (see above).
- Rinse 5×2 min in PBS.
Permeabilization Step
- Incubate the slides with 0.25-0.5% Triton X-100 (or other permeabilization medium) in PBS for 10 minutes to permeabilize the membranes (Note: there is no need for a permeabilization step following acetone or methanol fixation).
- Rinse 3×5 min in PBS.
Endogenous Peroxidase Blocking Step
- Block endogenous peroxidase by incubating in 3% H2O2 in PBS for 10-30 minutes (This step is required only if an HRP conjugated secondary antibody is to be used.)
- Rinse in 3x5min in PBS.
Blocking of Non-specific Binding
- Block with 2-5% normal serum in PBS for 1 hour. Normal serum should be the same species from which the secondary antibody was raised. (Alternatively, 5% BSA is sometimes used as blocking agent.)
Primary Antibody Incubation
- Dilute the primary antibody to the recommended concentration in 1% normal serum, PBS.
- Remove blocking buffer from the slides.
- Add primary antibody to each well. Incubate for 1-2 hours at RT or overnight at 4°C.
- Remove the primary antibody solution and rinse slides 3 x 5 minutes in PBS.
Secondary Antibody Incubation
- Dilute the biotinylated secondary antibody in 1% BSA diluent.
- Remove the excess fluid from the slide and add secondary antibody solution into each well. Incubate for 1 hour at RT.
- Rinse 3 x 5 minutes in PBS. Remove excess fluid.
Color Development
- Add streptavidin-HRP to each well. Incubate for 30 minutes at RT.
- Wash 3 x 5 minutes in PBS on an orbital shaker. Remove excess fluid.
- Add DAB solution to each cell well. Once the cells start turning brown (this can be observed under a microscope) wash 2 x 5 minutes in PBS on the shaker.
Optional Counterstain
- Dip the slides into a staining dish of hematoxylin for 30 seconds.
- Remove and place into an acid bath (200ml dH2O and 1-3 drops of acetic acid). Rinse with dH2O.
Cover Slips
- Add cover slips to the slides for examination under the microscope