Dye-labeled peptides and oligonucleotides are important tools in biochemical and cellular studies. Fluorescent peptides and oligonucleotides have been extensively used in all major types of fluorescence imaging including fluorescence resonance energy transfer (FRET). These labeled biomolecules are widely used for diagnosing infectious diseases based on the molecular beacon and other technologies. FRET peptides and oligonucleotides have been also used for cell analysis via fluorescence-associated cell sorting (FACS) either in vivo or in vitro for research and diagnostic purposes.
Peptide and Oligonucleotide Labeling
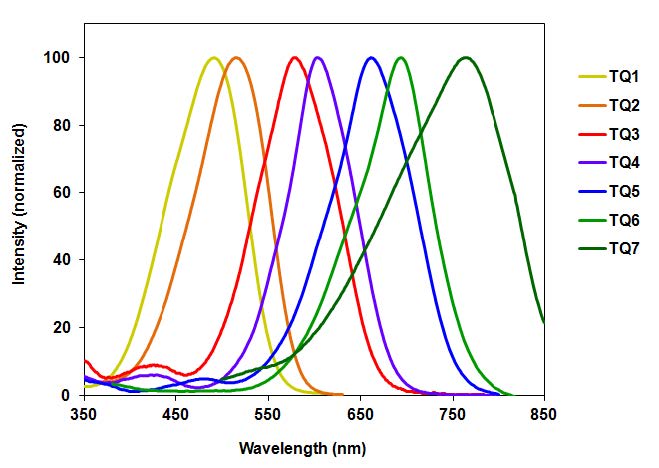
The most important characteristics of dye-labeled peptides and oligonucleotides are high sensitivity and non-radioactive detection. Using suitable quenchers, either classic or modern (such as the Tide Quencher™ series in the image above) can assist researchers with superior non-fluorescent imaging.
Peptides and Oligonucleotides
Peptides are short chain amino acids that perform numerous functions as chemical messengers, hormones, cellular mediators, highly specific stimulators and inhibitors, as well as novel therapeutic treatments for various diseases including Alzheimer’s and cancer. Due to the emerging solid-phase peptide synthesis technologies, synthetic peptides have now been widely used in biochemistry, molecular biology, and pharmaceutical areas. Dye-labeled peptides are powerful tools to investigate cellular structure, analyze receptor-ligand or protein-protein interactions, study cellular transport, and measure enzymatic activity via FRET analysis.
Fluorescent Labeling Dyes
A fluorescent dye can be attached to a peptide/an oligonucleotide at a specific point through a covalent bond depending on the sequence of peptide/oligonucleotide. The linkage between dye and peptide/oligonucleotide is a covalent bond, which is stable and not destructive under most biological conditions. In some cases, a functional linker is introduced between dye and peptide/oligonucleotide to minimize the alteration of peptide/oligonucleotide biological activity. For all the peptide/oligonucleotide labelings, the dye need be attached at a defined position. In general, the preferred fluorescent labels should have high fluorescence quantum yields and retain the biological activities of the unlabeled biomolecules. AAT Bioquest offers a variety of fluorescent labeling dyes for facilitating the conjugation of dyes to peptide/oligonucleotide that are used for biological studies. These fluorescent dyes include coumarins, fluoresceins, rhodamines and cyanines. Among them, our Tide Fluor™, California Red™, SunRed™ and Helix Fluor™ dyes are optimized for labeling peptides and oligonucleotides.
Cyanine Dye Family and Other Classic Dyes
Although modern dyes such as iFluor™ dyes are often used for the more challenging biological applications, the classic fluorescent labeling dyes are sometimes preferred for some less demanding applications due to their significantly lower costs. Among the classic labeling dyes, cyanines are one of the most popular choices. Other options include FAM, TAMRA, and Texas Red® for preparing peptide and oligonucleotide conjugates.
- ?: is the extinction coefficient (cm-1M-1).
- ?: is the fluorescence quantum yield.
Tide Fluor™ Series
Tide Fluor™ dyes are optimized as building blocks for developing FRET oligonucleotides and peptides. Our Tide Fluor™ dyes have stronger fluorescence and higher photostability than the classic labeling dyes such as coumarins, fluoresceins, rhodamines and cyanines. They are the best affordable fluorescent dyes for labeling peptides and oligonucleotides without sacrificing performance.
Table 2. Tide Fluor™ Series For Fluorescent Labeling Dyes
| Labeling Dye | Abs (nm) | Em (nm) | ε1 | Φ2 | CF at 260 nm3 | CF at 280 nm4 |
| Tide Fluor™ 1 | 345 | 442 | 20000 | 0.95 | 0.246 | 0.187 |
| Tide Fluor™ 2WS | 502 | 525 | 75000 | 0.9 | 0.211 | 0.091 |
| Tide Fluor™ 2 | 500 | 527 | 75000 | 0.9 | 0.288 | 0.201 |
| Tide Fluor™ 3WS | 555 | 565 | 150000 | 0.105 | 0.079 | 0.079 |
| Tide Fluor™ 3 | 555 | 584 | 85000 | 0.85 | 0.331 | 0.201 |
| Tide Fluor™ 4 | 590 | 618 | 90000 | 0.91 | 0.489 | 0.436 |
| Tide Fluor™ 5WS | 649 | 664 | 250000 | 0.25 | 0.023 | 0.027 |
| Tide Fluor™ 6WS | 676 | 695 | 220000 | 0.18 | 0.111 | 0.009 |
| Tide Fluor™ 7WS | 749 | 775 | 275000 | 0.12 | 0.009 | 0.049 |
| Tide Fluor™ 8WS | 775 | 807 | 250000 | 0.08 | 0.103 | 0.109 |
- ? = Extinction coefficient (cm-1M-1) at their maximum absorption wavelength
- ? = Fluorescence quantum yield in aqueous buffer (pH 7.2)
- CF at 260 nm is the correction factor used for eliminating the dye contribution to the absorbance at 260 nm (for oligos and nucleic acid labeling)
- CF at 280 nm is the correction factor used for eliminating the dye contribution to the absorbance at 280 nm (for peptide and protein labeling)
Other Proprietary Series
AAT Bioquests’ California Red™, Sunnyvale Red™ and Helix Fluor™ dyes are ideally suited for robust, efficient labeling of oligonucleotides as well as peptides. These series, with improved performance and brightness, are still suitable for all major instrumentation platforms and lasers as used with older fluorescent labeling dyes such as FAM, TET, VIC, JOE, NED, TAMRA and ROX.
Table 3. Other Proprietary Series For Fluorescent Labeling Dyes
| Cat# | Product Name | Abs (nm) | Em (nm) | Unit Size |
| 250 | Helix Fluor™ 545, succinimidyl ester | 525 | 545 | 1 mg |
| 251 | Helix Fluor™ 575, succinimidyl ester | 546 | 575 | 1 mg |
| 394 | Sunnyvale Red™ SE *Superior 6-ROX Replacement* | 576 | 605 | 5 mg |
| 473 | California Red™ SE | 583 | 603 | 5 mg |
| 479 | California Red™ SE | 583 | 603 | 1 mg |
Non-Fluorescent Labeling Dyes
Proteases are involved in a number of physiological processes. The detection of proteases and the screening of specific protease inhibitors are essential in the discovery of potential drugs for the treatment and management of protease-related diseases. A variety of donor and acceptor molecules (either fluorescent or non-fluorescent) are attached to the peptide(oligo)/protein (forming the internally quenched conjugates), and are used to detect a protease depending on the sequences of the amino acids. The donor and acceptor molecules are carefully chosen so that the absorption of the acceptor overlaps with the fluorescence of the donor, thus ensuring that the fluorescence is quenched through resonance energy transfer. Enzyme hydrolysis of the substrate results in spatial separation of the donor and the acceptor molecules, thereby restoring the donor’s fluorescence.
FRET Mechanism
Förster resonance energy transfer (FRET) is a physical phenomenon that has been gaining popularity in biomedical research and drug discovery. FRET is the heatless transmission of energy from a donor molecule to an acceptor molecule. The donor molecule is the dye or chromophore that initially absorbs the energy and the acceptor is the chromophore to which the energy is subsequently transferred. This resonance interaction occurs over greater than interatomic distances, without conversion to thermal energy and without any molecular collision. The transfer of energy leads to a reduction in the donor’s fluorescence intensity and excited state lifetime, and an increase in the acceptor’s emission intensity. A pair of molecules that interact in such a manner that FRET occurs is often referred to as a donor/acceptor pair. Due to its sensitivity to distance, FRET has been used to investigate molecular interactions.
While there are many factors that influence FRET, the primary conditions that need to be met in order for FRET to occur are relatively few:
- The donor and acceptor molecules must be in close proximity to one another (typically 10-100 Å).
- The absorption or excitation spectrum of the acceptor must overlap the fluorescence emission spectrum of the donor. The degree to which they overlap is referred to as the spectral overlap integral (J).
- The donor and acceptor transition dipole orientations must be approximately parallel.
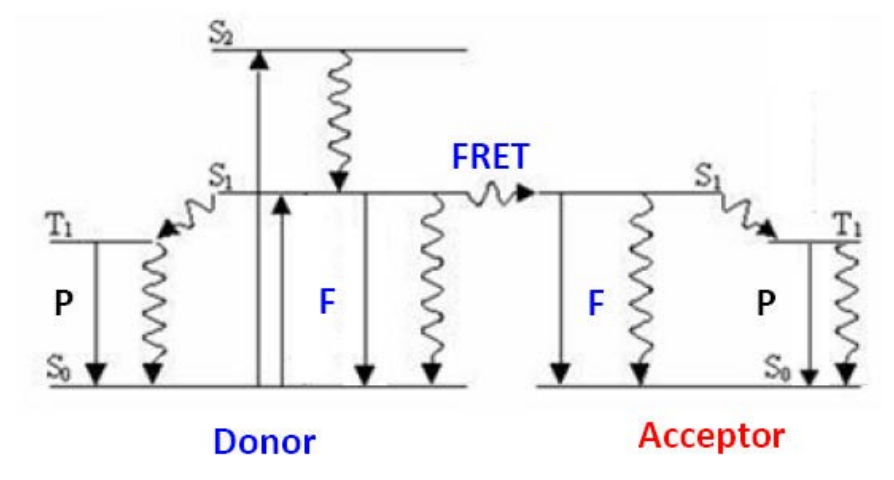
Assuming that the donor acceptor pairs are compatible, the most critical element necessary for FRET to occur is close proximity of the pairs. Förster demonstrated that the efficiency of the process (E) depends on the inverse sixth power distance between donor and acceptor as expressed with the following equation:
E = Ro6/(Ro6 + r6)
Where Ro is the Förster distance at which half the energy is transferred and r is the actual distance between donor and acceptor. The magnitude of the Ro is dependent on the spectral properties of the donor and the acceptor. Förster distances ranging from 20 to 90 Å are most useful for the studies of biological macromolecules.
Tide Quencher™ Series
Although DABCYL has been used to develop a variety of FRET applications, its low quenching efficiency for longer wavelength dyes (such as fluoresceins, rhodamines and cyanines) has limited its use in the development of sensitive fluorogenic FRET probes. Additionally, the absorption spectrum of DABCYL is environmentsensitive. AAT Bioquest has developed robust Tide Quencher™ acceptor dyes for the development of longer wavelength FRET probes. Tide Quencher™ dyes are a great choice to eliminate the limitations of classic quenchers.
The Key Benefits of Tide Quencher™ Dyes:
- Explore the FRET potentials that might be impossible with other quenchers.
- Versatile reactive forms are convenient for self-constructing your desired FRET biomolecules.
- Perfectly match your desired fluorescent donors.
- Competitive price with better performance.
Table 4. Tide Quencher™ Series For Non-Fluorescent Labeling Dyes
| Quencher | Abs (nm) | ε*1 | CF at 260 nm*2 | CF at 280 nm*3 |
| Tide Quencher™ 1 | 488 | 20,000 | 0.147 | 0.194 |
| Tide Quencher™ 2 | 512 | 21,000 | 0.1 | 0.12 |
| Tide Quencher™ 2WS | 515 | 21,000 | 0.1 | 0.12 |
| Tide Quencher™ 3 | 576 | 22,000 | 0.085 | 0.091 |
| Tide Quencher™ 3WS | 576 | 90,000 | 0.186 | 0.205 |
| Tide Quencher™ 4 | 604 | 23,000 | 0.146 | 0.183 |
| Tide Quencher™ 4WS | 604 | 90,000 | 0.149 | 0.136 |
| Tide Quencher™ 5 | 661 | 24,000 | 0.17 | 0.082 |
| Tide Quencher™ 5WS | 661 | 130,000 | 0.072 | 0.082 |
| Tide Quencher™ 6WS | 691 | 130,000 | 0.12 | 0.102 |
| Tide Quencher™ 7WS | 759 | 140,000 | 0.072 | 0.091 |
- ? = Extinction coefficient (cm-1M-1) at their maximum absorption wavelength
- CF at 260 nm is the correction factor used for eliminating the dye contribution to the absorbance at 260 nm (for oligos and nucleic acid labeling)
- CF at 280 nm is the correction factor used for eliminating the dye contribution to the absorbance at 280 nm (for peptide and protein labeling)