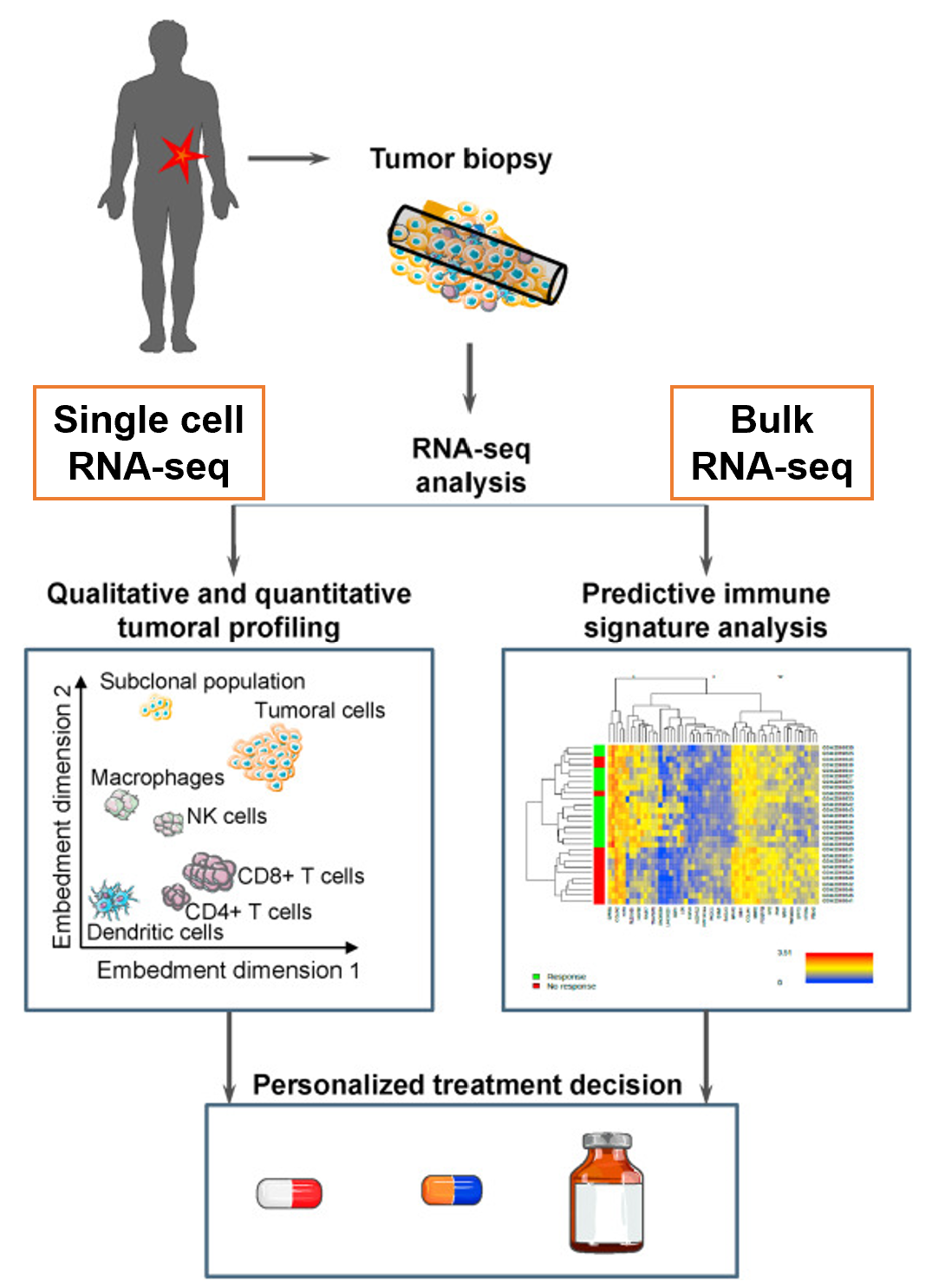
Tumours are very heterogeneous. Somewhat counterintuitively, this heterogeneity goes beyond the malignant cells. In fact, tumours are also made up and influenced by non-malignant cells, such as stromal and immune cells [1].
This is why the scientific community is progressively getting interested not only in profiling the genetic alterations that occur in tumours, but also in profiling and understanding the mechanisms by which immune cells drive and control tumour’s progression.
Immunotherapy itself is an approach that aims at using or re-purposing a patient’s immune system to fight back cancer.
As for all therapies – and in particular the ones targeting complex diseases such as cancer – the efficacy of immunotherapy can greatly be improved by personalizing and tailoring treatments to patients. However, this is unfortunately easier said than done!
In fact, the scientific and medical community still lack reliable biomarkers and interpretation tools to be able to reliably personalize immunotherapy.
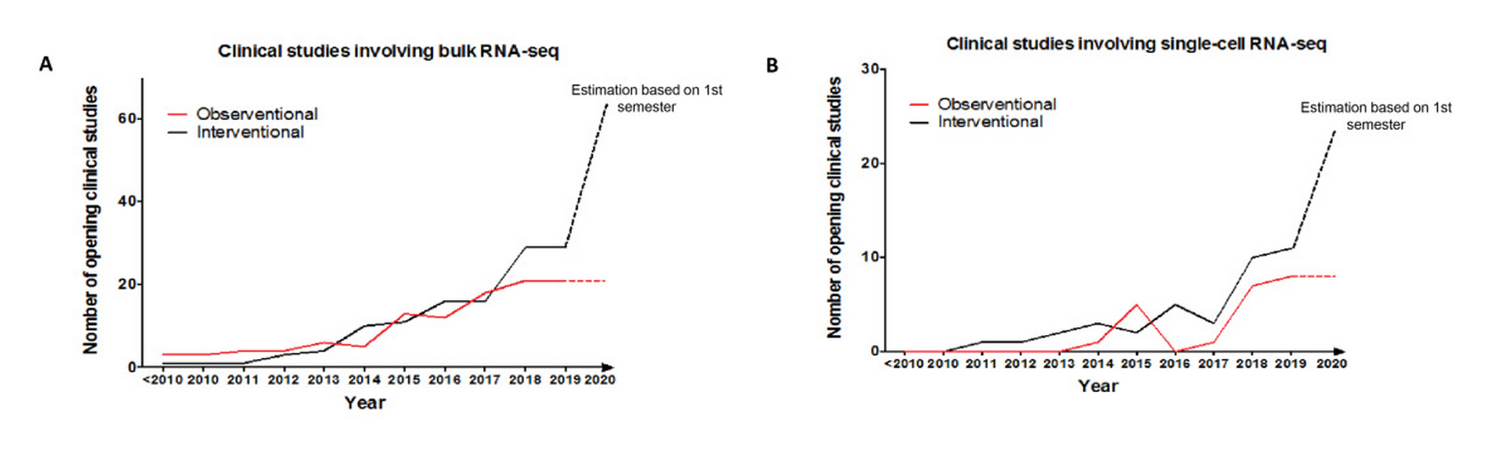
Luckily, accumulating evidence and emerging tools are opening exciting opportunities.
In this context, both bulk and single-cell RNA sequencing (RNA-seq) constitute great resources for the development of new knowledge and new biomarkers that can help provide the right therapy at the right time (Fig. 1).