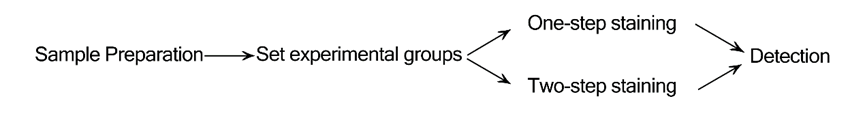
This video is about how to operate Elabscience® Annexin V Apoptosis Detection Kit. For each reaction, there is a demonstration, so that the experimenters can better understand this experiment process. In addition to the traditional one-step staining, this video also shows Elabscience’s unique two-step staining method, which can help you save reagents.
Elabscience Technical Videos
This video is about how to operate Elabscience® One-step TUNEL experiment by Flow Cytometry. For each reaction, there is a demonstration, so that the experimenters can better understand the Flow Cytometry TUNEL process.
Procedure:
Sample Preparation → Count the cells → Fixation → Permeabilization → Equilibrium → Labeling → Stop reaction → Detection
This video shows the routine operation process of human peripheral blood PBMC preparation, mainly shows the method of extracting PBMC with Ficoll separation solution, and notes the details that need attention in the experiment, so as to better help the experimenter understand the PBMC sample preparation experiment.
Attention: The operation procedure of Ficoll separation solution produced by different manufacturers may be different. Please refer to the instruction manual during the experiment
This is an easy tutorial about cell intracellular targets staining for flow cytometry, this video shows the experiment procedure of flow cytometry such as: Block Fc Receptor, Set Sample and Control, Cell Surface Staining, Fixation and Permeabilization, so that the experimenters can better understand this experiment.
Procedure:
Sample Preparation → Cell Counting → Block Fc Receptor(optional) → Cell Surface Staining → Fixation and Permeabilization → Cell Intracellular Staining → Detection → Analysis
This is an easy tutorial about cell surface targets staining for flow cytometry, this video shows the experiment procedure of flow cytometry such as: Block Fc Receptor, Set Sample and Control, Cell Surface Staining, so that the experimenters can better understand this experiment.
Procedure:
Sample Preparation → Cell Counting → Block Fc Receptor(optional) → Cell Surface Staining → Detection → Analysis
This is an easy tutorial about cell surface targets staining for flow cytometry, this video shows the experiment procedure of flow cytometry such as: Block Fc Receptor, Set Sample and Control, Cell Surface Staining, so that the experimenters can better understand this experiment.
Procedure:
Sample Preparation → Cell Counting → Block Fc Receptor(optional) → Cell Surface Staining → Detection → Analysis
This video is about how to operate Super Plus High Sensitive and Rapid Immunohistochemical Kit. For each reaction, there is a demonstration, so that the experimenters can better understand the IHC process and how to operate this kit.
Super Plus High Sensitive and Rapid Immunohistochemical Kit
Elabscience® Super Plus TMHigh Sensitive and Rapid Immunohistochemical Kit is a high sensitive and rapid immunohistochemical broad spectrum detection reagent. There is no need to do the traditional three times of xylene dewaxing, three to five times of gradient ethanol hydration or antigen repair but only one Dewaxing/Antigen Retrieval Buffer heating without fume hood or traditional dewaxing/antigen repair tanks. The operation time is effectively shortened, the cumbersome operation steps are simplified so the variables in operation are reduced, and the stability is improved. The Dewaxing/Antigen Retrieval Buffer applies new environmental protection technology is not only reduces the harm to the body, but also cause no pollution to the environment.
This kit is easy to operate and fast because of the one-stop reagents used in the immunohistochemical experiment, and the high sensitive secondary antibody/ DAB detection reagents make this kit to be more sensitive than other produces.
This kit can be used to detect monoclonal/polyclonal antibody derived from Rabbit and Mouse.
This video is about how to operate Elabscience® One-step TUNEL (Paraffin Section) experiment. For each reaction, there is a demonstration, so that the experimenters can better understand the TUNEL process.
Procedure:
Paraffin Section → Dewaxing → Washing → Permeabilization → Washing → Equilibrium → Labeling → Washing → Nuclear staining → Washing → Mounting → Analyze sample
This video is about how to operate Elabscience® One-step TUNEL (Cell Slide) experiment. For each reaction, there is a demonstration, so that the experimenters can better understand the TUNEL process.
Procedure:
Cell Slide → Fixation → Washing →Permeabilization → Washing → Equilibrium → Labeling → Washing → Nuclear staining → Washing → Mounting → Analyze sample
Elabscience® has developed a series of fluorometric assay kits with simple operation, high sensitivity and excellent quality through the method of enzyme reaction with fluorescent probe.
This video provides a brief introduction to operation of fluorometric assay kit to help you better understand the process from detecting process to result analysis.
This is a video about Preparation of Animal Tissue Samples for Metabolism Assays.
Take 0.02-1g fresh tissue to wash with homogenization medium at 2-8℃ to remove blood cells. Absorb the water with filter paper and weigh. Homogenize at the ratio of the volume of homogenized medium (2-8℃) (mL): the weight of the tissue (g) =9:1, then centrifuge the tissue homogenate for 10 min at 10000 g at 4℃. Take the supernatant to preserve it on ice for detection.
This video is the operation guiding for cell ferrous iron colorimetric assay kit, using cell samples for the assay.
The video shows the whole process of the assay, including sample preparation, reagent preparation, operation process and data analysis, helps you to have a better understanding for the detection of cell ferrous iron concentration.
This video is the operation guiding for cell ferrous iron colorimetric assay kit, using cell samples for the assay.
The video shows the whole process of the assay, including sample preparation, reagent preparation, operation process and data analysis, helps you to have a better understanding for the detection of cell ferrous iron concentration.
This is a video about Food Safety Kit Operation Guide Video.
Test principle
Elabscience offers food safety test kits with competitive-method which can detect toxin, residues and contaminants. Our food safety testing kits are suitable for a variety of samples, such as cereals, tissue, honey, milk, edible oil, etc. The microtiter plate in this kit has been pre-coated with coupled antigen. During the reaction, target in the samples or standard competes with coupled antigen on the solid phase supporter for sites of anti-antibody. Then horseradish peroxidase (HRP) conjugate is added to each microtiter plate well, and substrate reagent is added for color development. There is a negative correlation between the OD value of samples and the concentration of target.
Procedure Overview
Solution preparation- Sample pretreatment procedure-Assay procedure- Result analysis.
Assay time: 5 min~ 75 min
This is an easy tutorial about western blot from Elabscience, this video provides tips to help you learn how to prepare samples, electrophoresis, transfer your proteins from the SDS-PAGE gel onto a PVDF, block the membrane,add the primary and secondary antibodies etc.
Procedure:
1、Samples preparation.
RIPA preparation: Prepare RIPA or NP40 buffer supplemented with fresh protease and phosphatase inhibitors. Prepare when needed. Calculate the total volume of RIPA buffer before processing the samples.
Tissue Processing: Cut the tissues into pieces on ice as quickly as possible to prevent degradation by proteases. Add 200-300ul of ice-cold RIPA lysis buffer for about 5mg of tissue samples, and homogenize on ice. Centrifuge at 12000rpm for 5 minutes at 4°C, then take the supernatant.
Adherent cell processing: Take out the culture medium and wash it with ice-cold PBS. Add 150uL of prepared lysis buffer for per 5×10^6 cells, then crack the mixture on ice for 5-10minutes and mix it with scraper. Take the disrupted sample into an EP tube. Centrifuge at 12000rpm for 5 minutes at 4°C, then take the supernatant.
Suspension cell processing: Transfer the cells into a pre-cooled centrifuge tube. Centrifuge at 2000-3000rpm for 5 minutes at 4°C, then discard the supernatant. Add ice-cold PBS to wash the cells. Centrifuge at 2000-3000rpm for 5 minutes at 4°C, discard the supernatant. Add 100-150ul of ice-cold RIPA lysis buffer for each 5×10^6 cells, then put the mixture on ice for 5-10minutes and mix it with pipette. Centrifuge at 12000rpm for 5 minutes at 4°C, then take the supernatant.
2、Protein concentration Measurement
Standard curve : Dilute the BSA with PBS. The recommended concentrations of BSA are 0, 0.2, 0.4, 0.6, 0.8, 1mg/ml. A duplicate is recommended for each concentration.
Dilute the tested samples with PBS to detect concentration.
Add 200uL of BCA reagents to the standard and sample wells, store out of light for 20-30 minutes at 37℃.
Determine the OD value at 568nm with microplate reader.
Calculate the protein concentration according to the average OD value of samples with the equation. Then calculate the loading volume of samples according to the protein concentration. The recommended total protein amount of each sample is 50ug.
Once you have determined the concentration of each sample, you can freeze them at -20°C or -70°C for later use or preparing for immune precipitation or loading into a gel.
3、Sample denaturation
Use a loading buffer with the anionic detergent sodium dodecyl sulfate (SDS) to denature the sample, then heat the mixture at 95–100°C for 10 minutes.
Dilute the sample supernatant with 5×SDS buffer at the ratio of 4:1. The best loading sample volume is 10ul and you must ensure that the loading volume is similar among the samples, so the sample should be diluted with PBS if the concentration is too high.
Heating in boiled water for 10 minutes.
Store the processed samples at -20℃(validity for 6 months), or at -70℃ for long-term storage.
4、SDS-PAG Gel preparation
Pour the mixed separating gel into the gel tank between two glass panes to about 50px below the top of the plastic holder.
Inject anhydrous ethanol or ddH2O above it to avoid affecting polymerization.
Incubate at 37℃ for 1 hour till it polymerizes completely, then pour out the ethyl alcohol.
Wash with ddH2O.
Absorb with filter paper, avoid touching the gel.
Add the stacking gel
Insert the comb carefully and avoid of forming bubbles.
Incubate at 37℃ for 1 hour till it polymerizes completely, then take out the comb.
The gel can be stored at room temperature for about 1 week in a sealed container with soaked towels to avoid of desiccation.
5、Electrophoresis
Add some electrophoretic buffer to the electrophoresis chamber, then fix the gel into the electrophoresis chamber. Make sure that there is no bubble on the platinum wire at the bottom of electrophoresis chamber.
Add electrophoretic buffer to the electrophoresis chamber. The buffer must be above the sample wells and the bottom of the gel must be immersed by buffer. Rinse all wells with electrophoretic buffer.
Add samples according to the calculated loading volume and add the protein marker.
Electrophoresis–The electrophoretic voltage of stacking gel is suggested to be 80V, lower than the electrophoretic voltage of separating gel which is suggested to be 110-150V generally in inconsecutive system/1. Samples should be at the same level when it reaches the separating gel. Electrophoresis time is about 2-3 hours till the bromophenol blue reaches the bottom of gel.
6、Electrotransfer (Wet transfer)
Take out the gel after the electrophoresis finished.
Cut the gel and wash it with ddH2O to remove the electrophoresis salts and detergents.
Prepare filter papers.
Soak the PVDF membrane in methanol for 5 seconds to re-activate it.
Use tweezers instead of fingers to avoid of touching the membrane.
Soak the PVDF membrane and filter paper and fiber mats into the electro transfer buffer.
Make sure that the PVDF membrane was cut into the same size as the gel. Large overhangs may prevent a current from passing through the membrane.
Put the related appliances in the following order, black plate – fiber mats – filter paper – gel -PVDF membrane – filter paper – fiber mat – white plate. After sandwiching the gel and membrane between filter papers, clean out the air bubbles between the gel and membrane with a roller, pipette or 15mL EP tube. Or you can assemble the sandwich device in transfer buffer to avoid of forming bubbles.
In a wet transfer device, the gel and membrane are sandwiched between sponge and filter paper and all these are tightly clamped together to ensure that no air bubbles will form between the gel and membrane. The sandwich device is submerged in transfer buffer in an electrical chamber. The negative-charged proteins will move towards the positively-charged electrode, so as to bind with the membrane.
Put the electrical chamber in an ice bath. Make sure that the PVDF membrane is near the positive pole.
Set a constant current. The electric current of each chamber is suggested to be 150-300mA. Adjust the transferring time according to molecular weight of protein. The ratio of SDS, methanol in the transfer buffer, protein size, and gel percentage may affect the transfer efficiency.
7、Blocking
Block the membrane to prevent the non-specific binding of the primary and secondary antibodies with the membrane.
Take out the PVDF membrane after transferring finished. Blot the transfer buffer up with filter paper.
Put the membrane into a blocking implement. Add either 5% skimmed milk -TBST solution or 5% BSA- TBST solution to block the membrane.
Milk is cheaper but is not recommended for studies of phospho-proteins. Milk contains casein, which is a kind of phospho-protein, which will cause high background because the phospho-specific antibody can detect the presence of casein in the milk.
Some antibodies produce a stronger signal on me membranes blocked with BSA opposed to milk for unknown reasons. Check the application notes on the datasheet in case there are specific instructions on how to block the membrane.
Incubate on the shaker for 2 hours at room temperature.
Membranes can be stored in TBST at 4℃ for up to 1 week.
8、Primary antibody Incubation
Take out the PVDF membrane after blocking. Blot the transfer buffer up with filter paper.
Add diluted primary antibody at the suggested dilution to the blocked blotting membrane. Too much antibody will result in non-specific bands. The primary antibody can be diluted with 5% milk-TBST.
The incubation time can vary from a few hours to overnight (rarely more than 18 h), which depends on the binding affinity of the antibody with the protein and the abundance of protein. We recommend a higher dilution ratio of antibody and prolong the incubation time to ensure the specific binding. If incubating overnight in blocking buffer, it is imperative to incubate at 4°C, otherwise contamination will occur and thus destruction of the protein will happen because of bacterial infection especially for phospho groups.
9、Membrane Washing
Remove the blocking buffer. Wash with TBST for 2 minutes to remove the residual primary antibody.
Wash the membrane with washing buffer on shaker for 5 minutes. Repeat the washing 3 times.
10、Secondary antibody Incubation
Take out the PVDF membrane after washing, blot the washing buffer up with filter paper.
Add the diluted peroxidase conjugated secondary antibody at the suggested dilution, and optimize the dilution according to the results. Too much antibody will result in non-specific bands. You may incubate the secondary antibody in blocking buffer, but a reduction in background may occur at the cost of a weaker specific signal, which presumably because the blocking protein hinders the binding of the antibody with the target protein.
Incubate the secondary antibody on shaker for 2 hours at room temperature.
We recommend the horseradish peroxidase (HRP)-conjugated secondary antibodies because Alkaline phosphatase (ALP)-conjugated secondary antibodies are less sensitive.
11、Membrane Washing
Remove the blocking buffer. Wash with TBST for 2 minutes to remove the residual secondary antibody.
Wash the membrane with washing buffer for on shaker for 5 minutes. Repeat the washing for 3 times.
12、Detection
The detection method depends on the conjugation of secondary antibody. ECL and DAB are usually used.
For HRP-conjugated antibodies, enhanced chemiluminiscence (ECL) kit is generally used as substrate
ECL preparation: Mix the substrate A and B at the ratio of 1:1.Prepare when needed.
Take out the PVDF membrane, and blot the washing buffer up with filter paper.
Cover the blotting membrane with mixed substrate for 1-5 minutes, and then observe the fluorescence in darkness with detection machine.
The camera detects the chemiluminescence emanating from the membrane, then transfer the signal into a digital image for rapid analysis with software provided with the detection machine.
Also we can use X-ray film to fixed the images or use DAB as chromogenic substrate when we use Alkaline phosphatase (ALP)-conjugated secondary antibodies.
13、Data analysis
Analyze the bands with Bandscan software.
This video is about how to operate antibody IHC experiment. For each reaction, there is a demonstration, so that the experimenters can better understand the IHC process.
Paraffin section immunohistochemical principle:
Fix the sample, then embed it into paraffin, dewaxing and microwave repair antigen of the embed tissue sections, add 3% hydrogen peroxide to block endogenous peroxidase, primary antibody combine with target protein, 4 ℃ incubation for the night, 37 ℃ rewarming for 30 min, wash clean by PBS and add secondary antibody incubation 35min (secondary antibody combine with primary antibody), wash clean then add chromogenic agent, secondary antibody’s tag react with chromogenic agent.After coloration add the stain nucleus with hematoxylin, 1% hydrochloric acid alcohol differentiation, washing back to blue then dehydration seal, air-dried then watch positive expression distribution under the mirror.
This is a video about Sandwich-ELISA Kit.
The micro ELISA plate provided in the kit has been pre-coated with an specific antibody. Standards or samples are added to the appropriate micro ELISA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for antigen and Avidin-Horseradish Peroxidase (HRP) conjugate is added to each micro plate well successively and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain antigen, biotinylated detection antibody and Avidin-HRP conjugate will appear blue in color. The enzyme-substrate reaction is terminated by the addition of a sulphuric acid solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The OD value is proportional to the concentration of antigen. You can calculate the concentration of antigen in the samples by comparing the O.D. of the samples to the standard curve.
This video is about how to operate Elabscience® TUNEL In Situ Apoptosis Kit (HRP-DAB Method). For each reaction, there is a demonstration, so that the experimenters can better understand the TUNEL process.
Procedure:
Paraffin Section → Dewaxing → Permeabilization → Blocking → Permeabilization →Equilibrium →Labeling and Developing → Nuclear staining → Mounting → Analyze sample
This video is about how to operate Elabscience® Enhanced Cell Counting Kit 8 (WST-8/CCK8) and analyze the experiment data. This video also shows the details that need to be paid attention to throughout the process. So that the experimenters can better understand this experiment process.
This is a video about QuicKey ProTM ELISA (Sandwich Method) Kit.
The micro ELISA plate provides in this kit has been pre-coated with antibody. Samples (or Standards) and HRP conjugate (enzyme-labeled antibody) are added to the micro ELISA plate wells at the same time. The antigen in samples (or standards) combines with the coated antibody and enzyme-labeled antibody to form a complex of the coated antibody-antigen- enzyme-labeled antibody. Then the free components are washed away from the plate. The substrate solution is added to each well. The enzyme-substrate reaction is terminated by the addition of stop solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450±2 nm, and the OD value is proportional to the concentration of the antigen. You can calculate the concentration of antigen in the samples by comparing the OD of the samples to the standard curve.
This is a video about QuicKey ProTM ELISA (Competitive Method) Kit.
The micro ELISA plate provided in this kit has been pre-coated with the antigen. Samples (or Standards) and HRP conjugate (enzyme-labeled antibody) are added to the micro ELISA plate wells at the same time. The antigen in samples (or standards) competes with a fixed amount of antigen on the solid phase supporter for sites on the enzyme-labeled antibody to form a complex of the coated antigen-enzyme-labeled antibody. Then the free components are washed away from the plate. The substrate solution is added to each well. The enzyme-substrate reaction is terminated by the addition of stop solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450±2 nm. The OD value is inversely proportional to the concentration of antigen. You can calculate the concentration of antigen in the samples by comparing the OD of the samples to the standard curve.
This is a video about QuicKey ELISA® Kit.
This ELISA kit uses the Sandwich-ELISA principle.The micro ELISA plate provides in this kit has been pre-coated with specific antibody. Samples (or standards) and biotinylated detection antibody specific are added to the micro ELISA plate wells at the same time. The antigen in the sample (or standard) would combine with the coated antibody, and the biotinylated detection antibody combine with the antigen bound to the coated plate. Free components are washed away. Then Avidin-Horseradish Peroxidase (HRP) conjugate are added successively to each micro plate well , biotin and avidin are specifically combined to form an immune complex. Free components are washed away. The substrate solution is added to each well. Only those wells that contain antigen, biotinylated detection antibody and Avidin-HRP conjugate will appear blue in color. The enzyme-substrate reaction is terminated by the addition of stop solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450 ± 2 nm. The OD value is proportional to the concentration of the antigen. You can calculate the concentration of the antigen in the samples by comparing the OD of the samples to the standard curve.