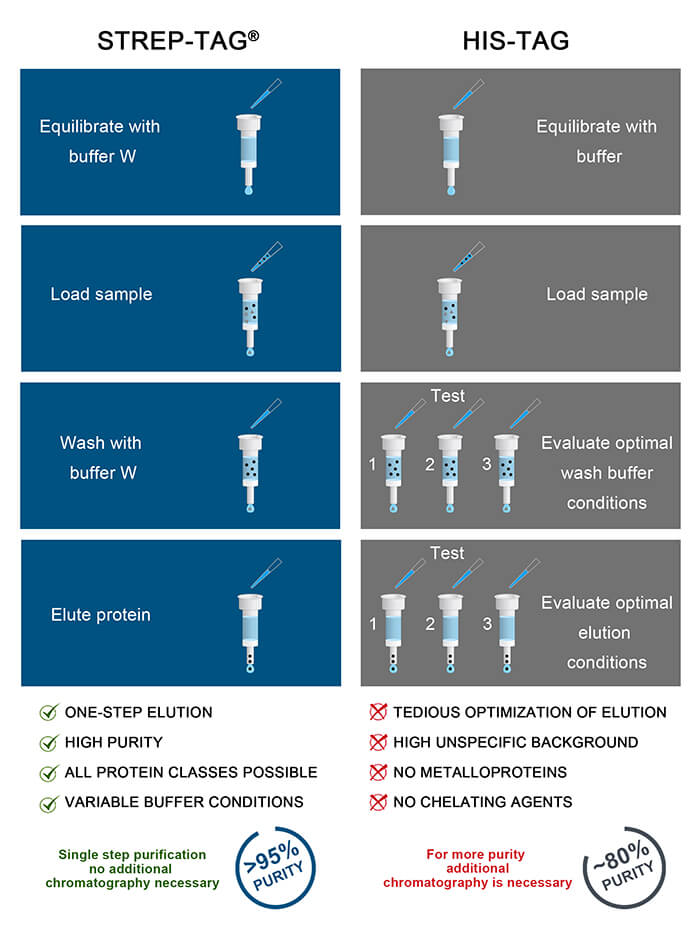
Strep-tag® performs better than His-tag in purification of mammalian-expressed proteins
In combination with high density mammalian protein expression, the His-tag system can lead to poor purification results, if conditions are not optimized. In order to achieve pure proteins for downstream applications from the coupling of the His-tag system and high density mammalian systems, further adjustments like dialysis of the supernatant, lower media:resin ratios or the use of strip-resistant nickel resins become necessary. Such adaptions require additional optimization time and effort, however, these challenges can be avoided by using the Strep-tag® system.
The following whitepaper shows the advantages of the Strep-tag® technology in comparison to the His-tag system as well as the excellent connection of the Strep-tag® technology with two high density mammalian expression systems, the Expi293TM and the ExpiCHOTM expression systems.
See why His-tag fails in some protein applications and learn more about the benefits and drawbacks of the two systems.
Strep-tag® – One affinity tag for all protein applications
By exploiting the highly specific interaction, Strep-tagged proteins can be isolated in one step from crude cell lysates in unparalleled purity. Because the Strep-tag® elutes under gentle, physiological conditions it is especially suited for generation of functional proteins e.g. enzymatic proteins. The mild (physiological) purification and elution conditions required for Strep-tag® fusion proteins make them suitable for structural and functional investigations, protein-protein interaction studies, ligand-receptor investigations or even separation of living cells for re-culturing processes. The system is suitable for multiple protein classes, e.g. metallo proteins, membrane proteins, fragile protein complexes with multiple subunits and any other protein class.
The near covalent affinity of Twin-Strep-tag®to Strep-Tactin®XT can be used to efficiently immobilize proteins for assay development. This makes the system to a universal platform and superior to all other affinity systems: one tag can be used for expression, purification, detection and immobilization.
For detailed information about further commonly used protein purification affinity tags we recommend the Editorial Article from Biocompare.
IBA is the original manufacturer of the Strep-tag® system and provides a complete portfolio around protein purification using this affinity system!