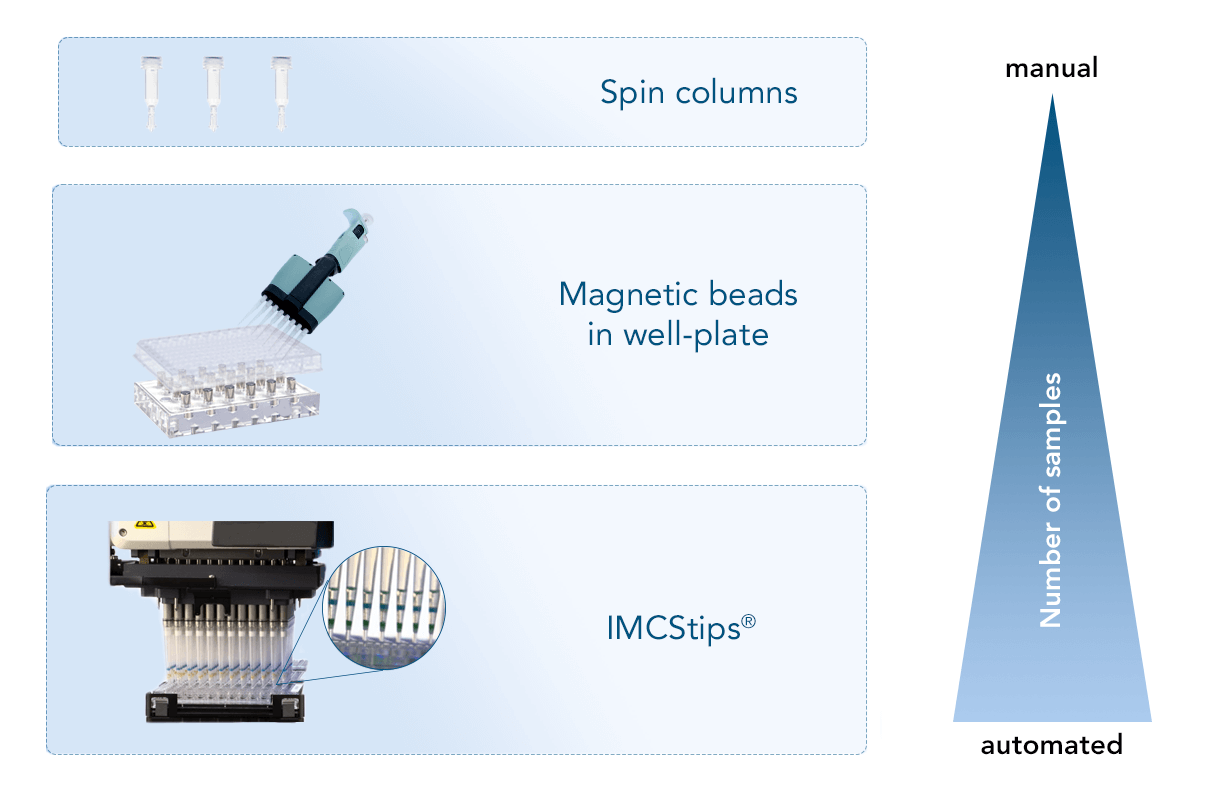
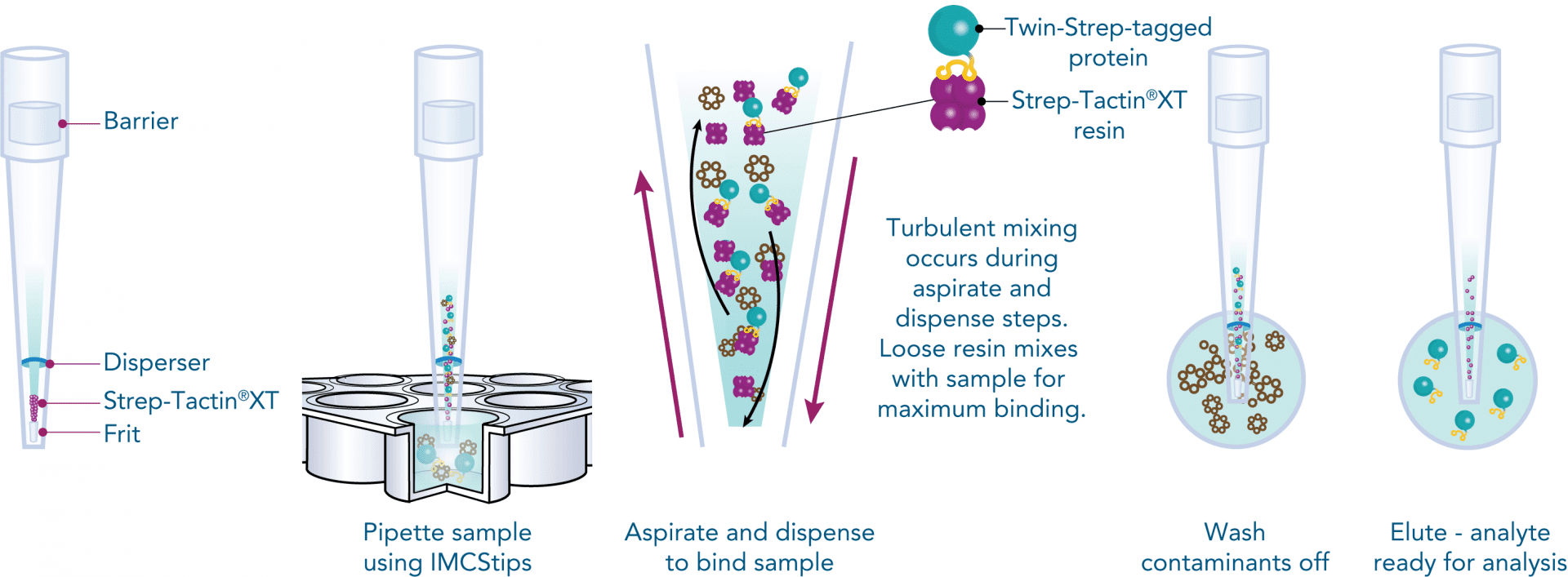
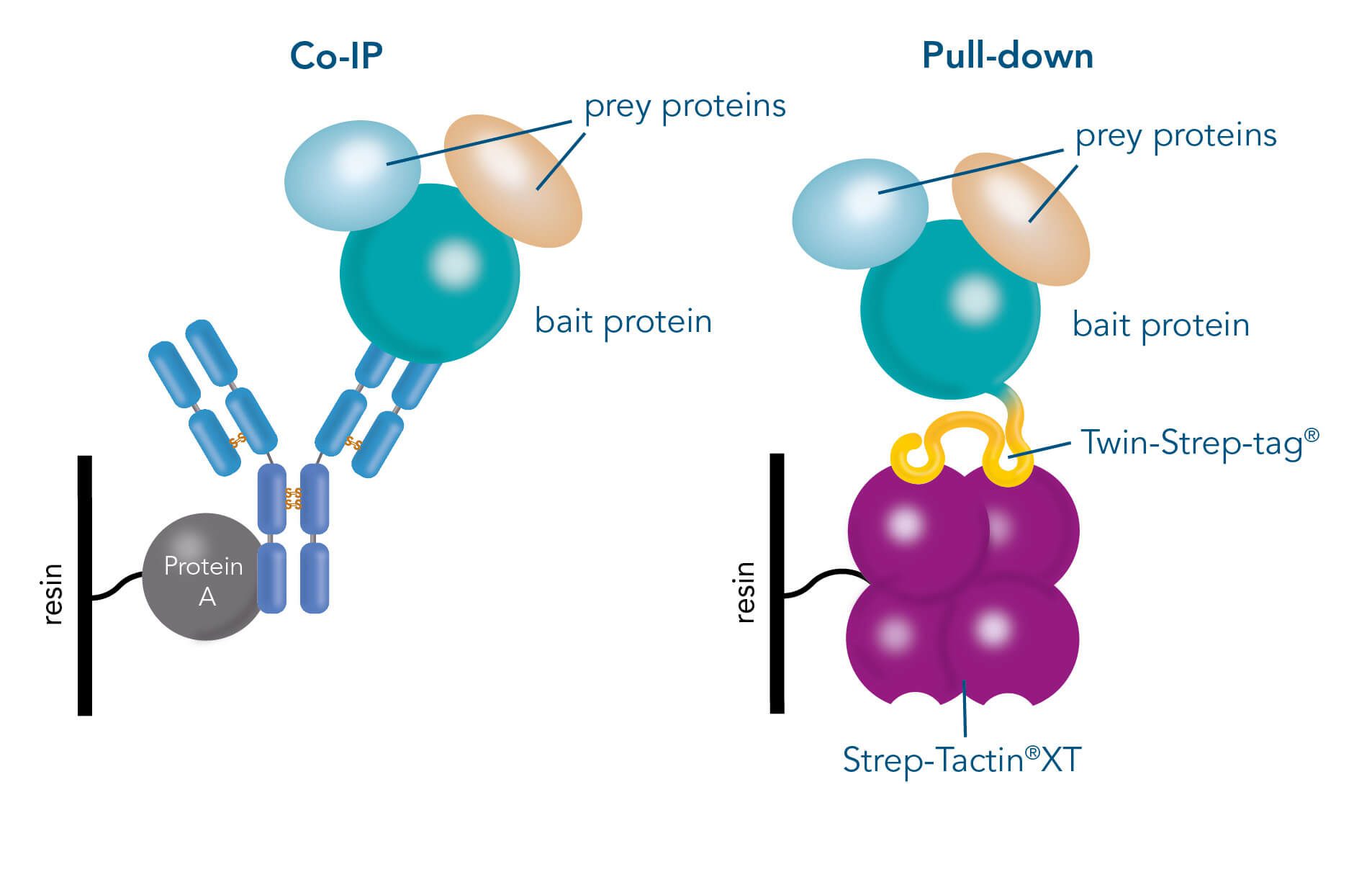
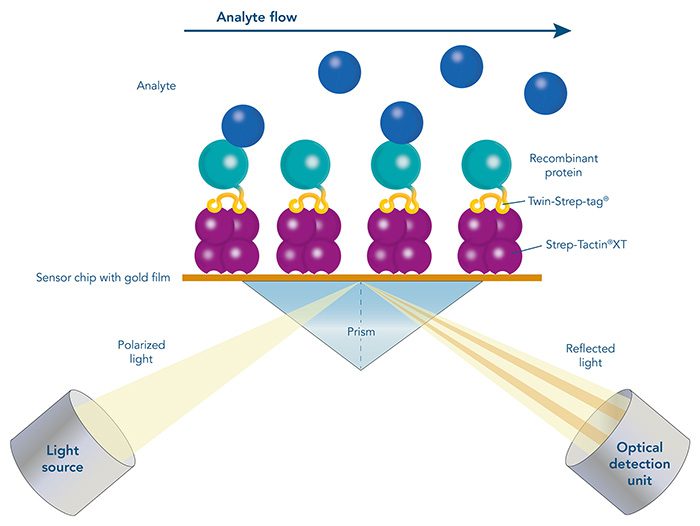
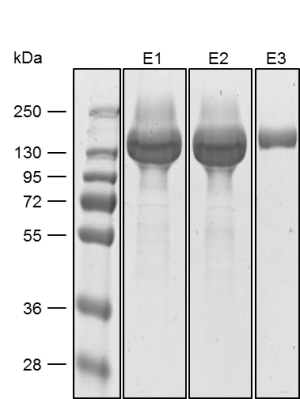
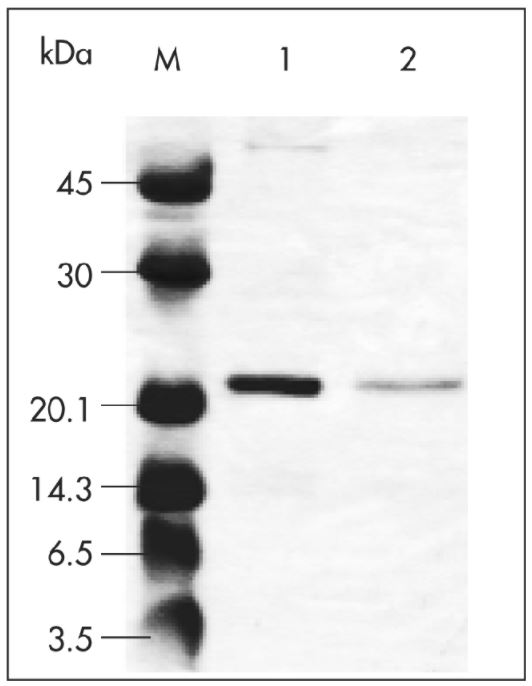
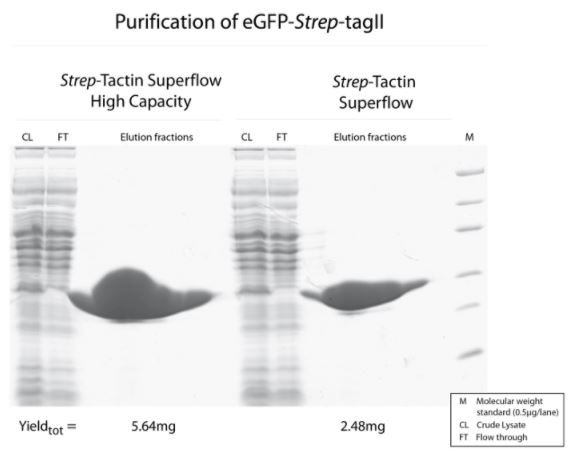
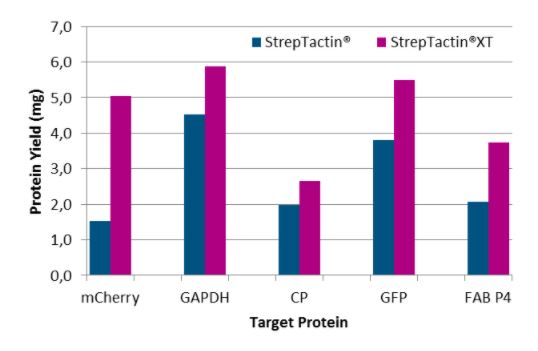
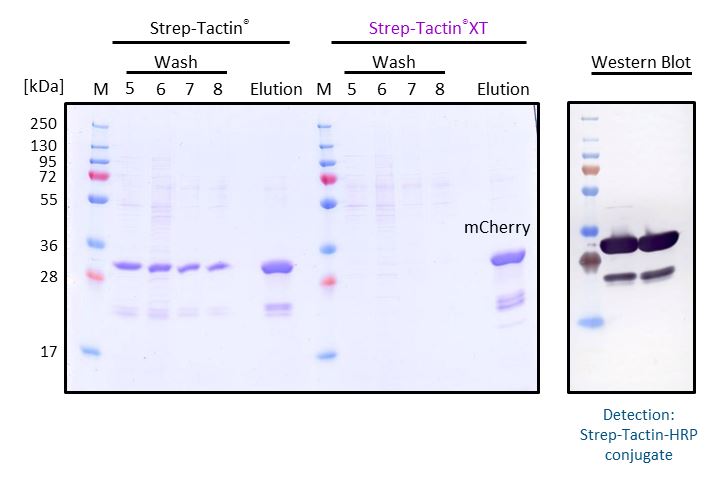
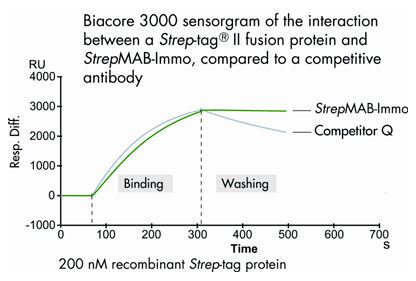
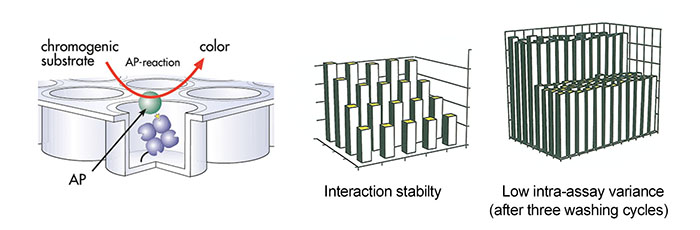
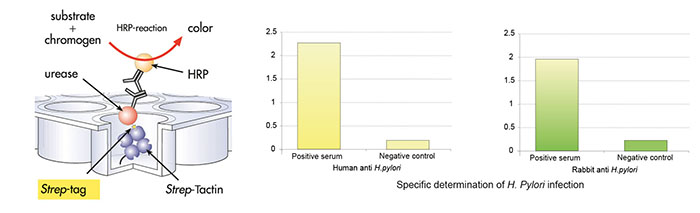
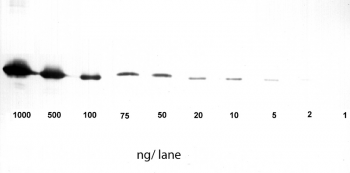
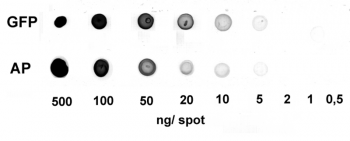
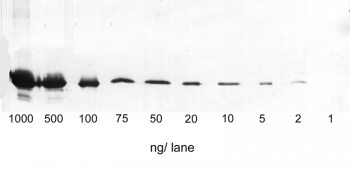
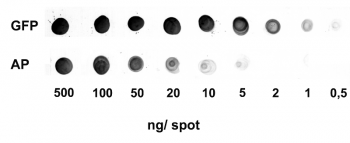
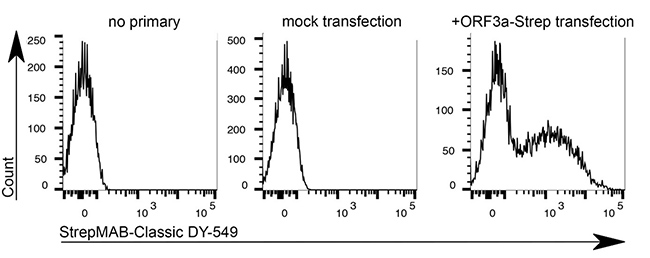
Antibody purification
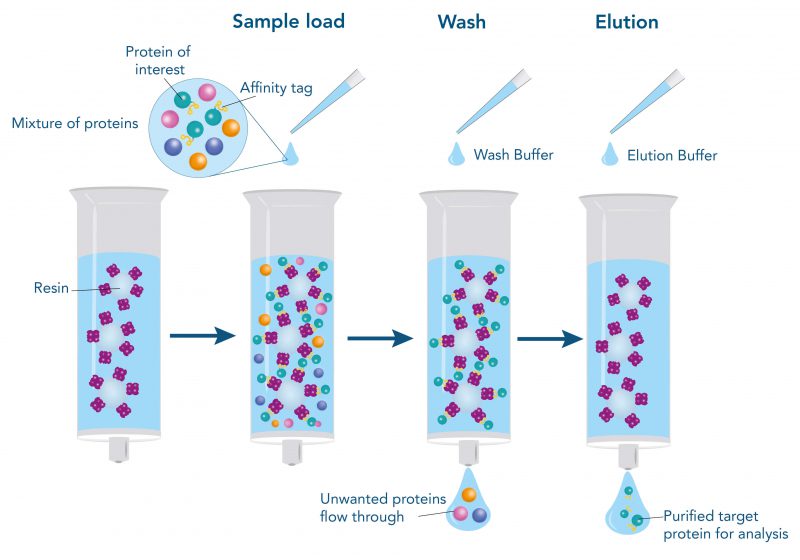
Antibodies are versatile tools in biotechnology. They can be utilized for the direct and indirect detection of antigens in various assays, as capture molecule immobilized on microplates, or as ligand for protein purification. However, before they can be deployed, they have to be purified from cell lysates, cell culture supernatants, or biological probes. The purification can take place without affinity tag via the physicochemical properties, antigen-specific affinity or the antibody class. Physicochemical properties are the molecular weight, charge, or clusters of specific residues. Depending on the origin of the sample, purification with the help of physicochemical properties or antigen-specific affinity can lead to the isolation of further proteins and antibodies with similar properties besides the target antibody. Thus, if a specific antibody with a high purity should be obtained, antibody class-specific affinity chromatography is recommended.
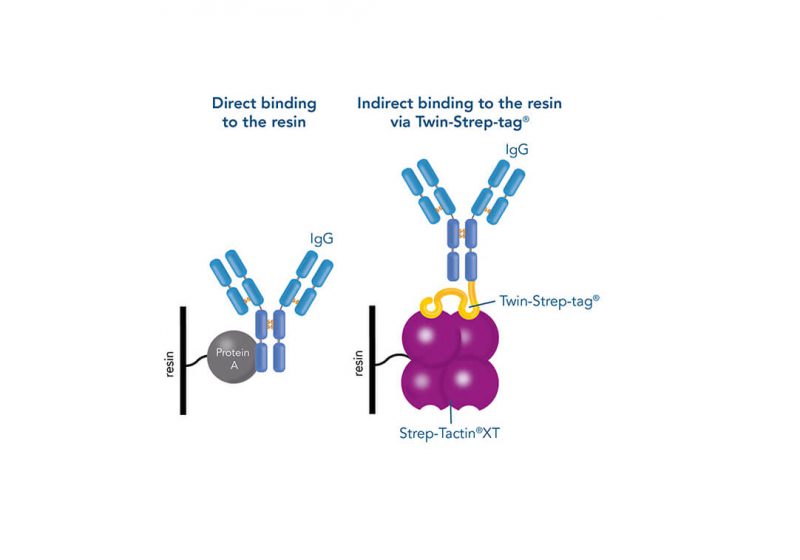
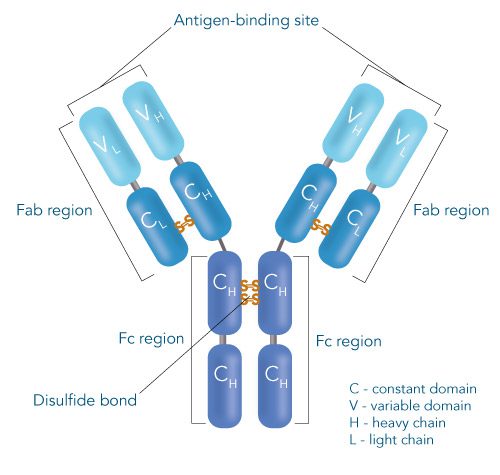
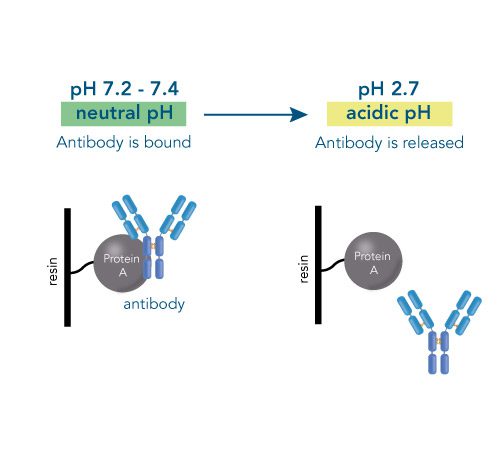
One of the ligands used for antibody class-specific affinity chromatography is Protein A. Originally, Protein A is a surface protein of the Staphylococcus aureus cell wall, which can bind immunoglobulins (antibodies) within the Fc region of their heavy chain without regard to antigen specificity. It is composed of five homologous Ig-binding domains that fold into a three-helix bundle. The IgG binding ability was utilized to produce an affinity tag-free system for direct purification of IgGs from serum, cell supernatants as well as extracts. The Protein A affinity chromatography resin captures IgGs from various mammalian species with different affinities under physiological buffer conditions (pH 7.2–7.4), whereas other molecules and antibodies flow through. Afterwards, the IgGs are released from capture by reduction of the pH to a more acidic value (pH 2.7).
Schematic structure of IgG antibodies
Principle of Protein A affinity chromatography
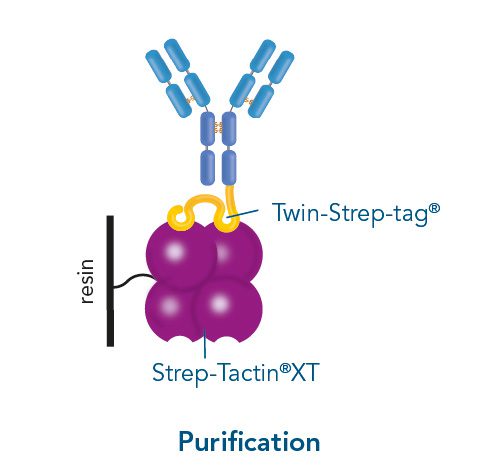
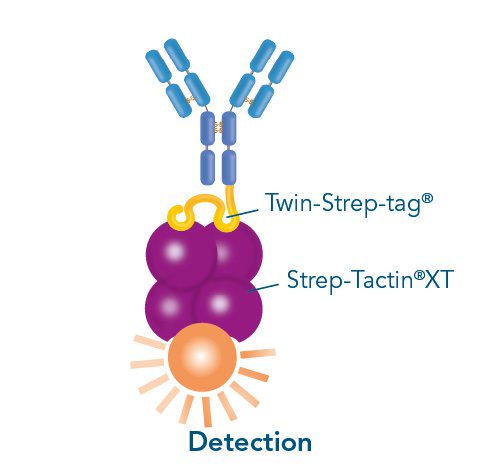
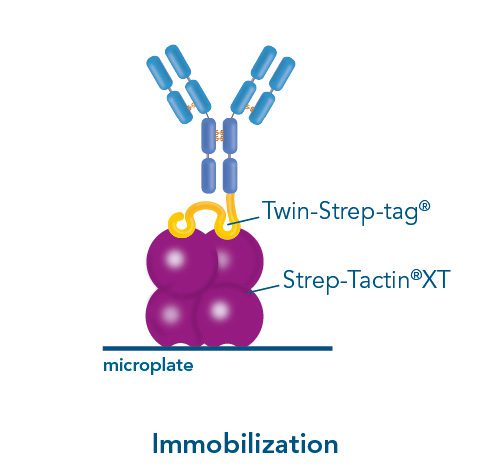
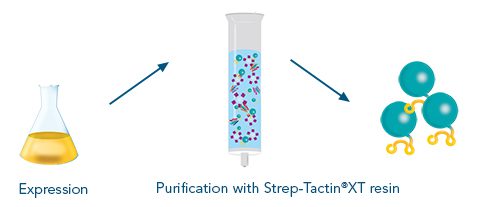
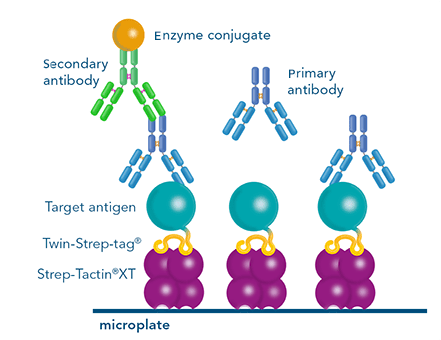
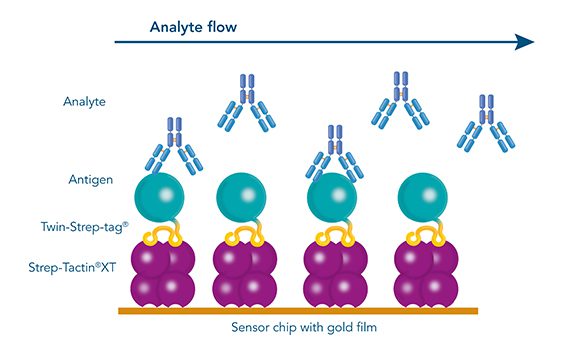
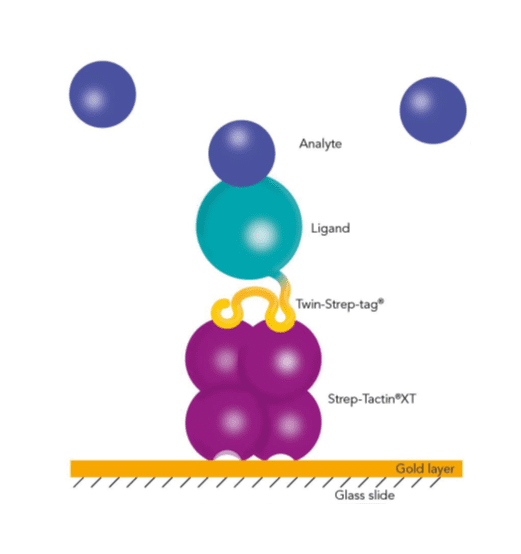
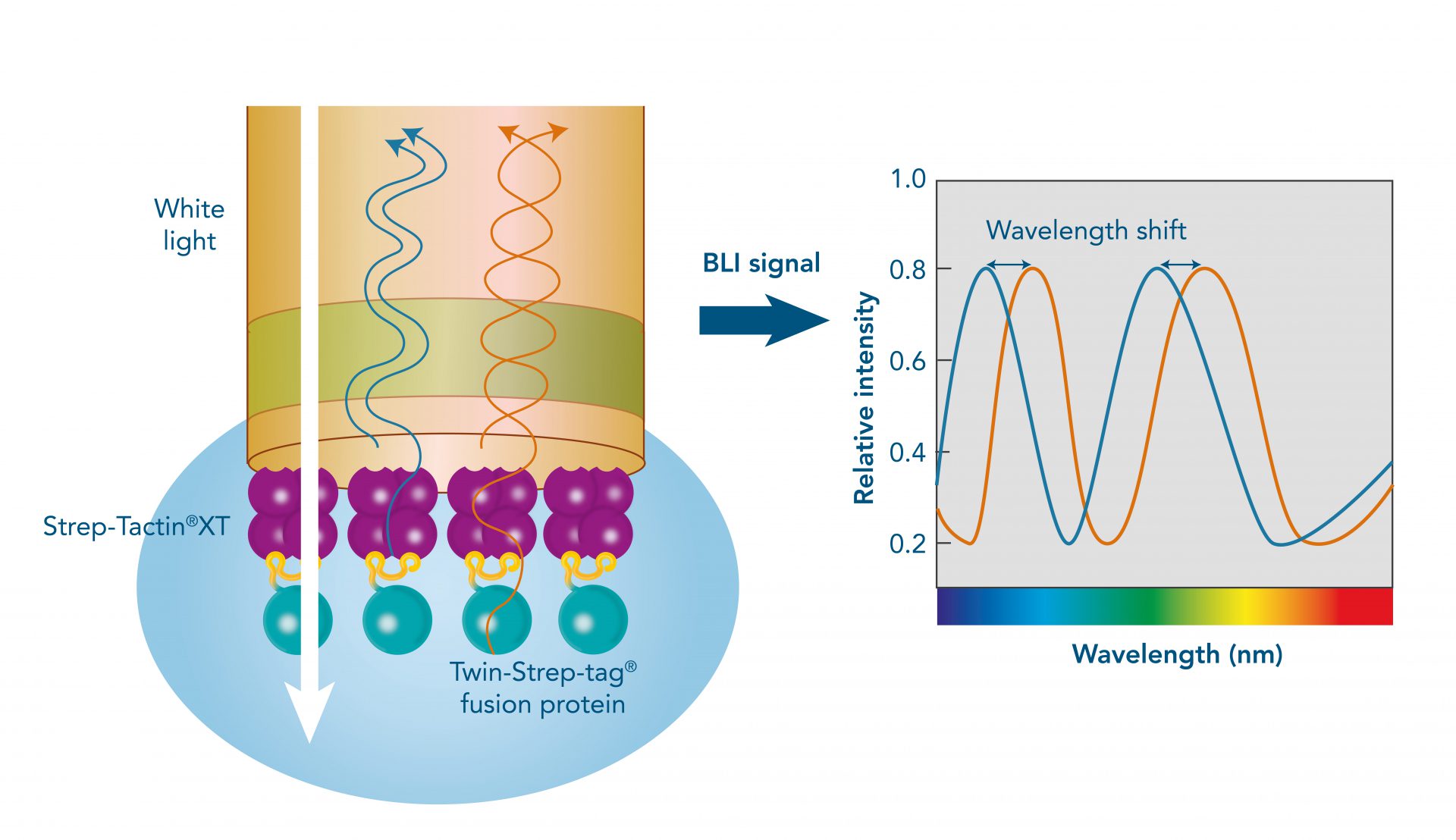
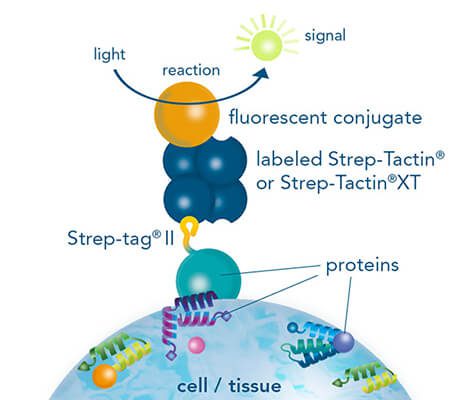
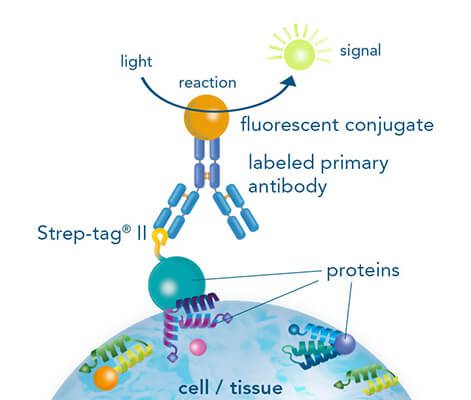
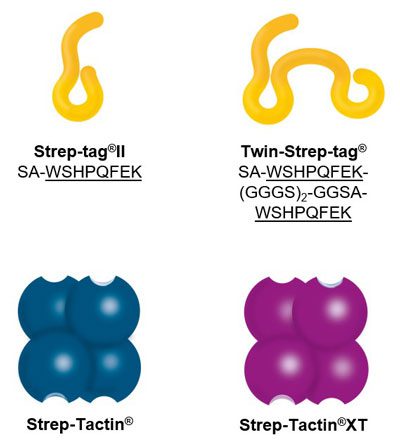
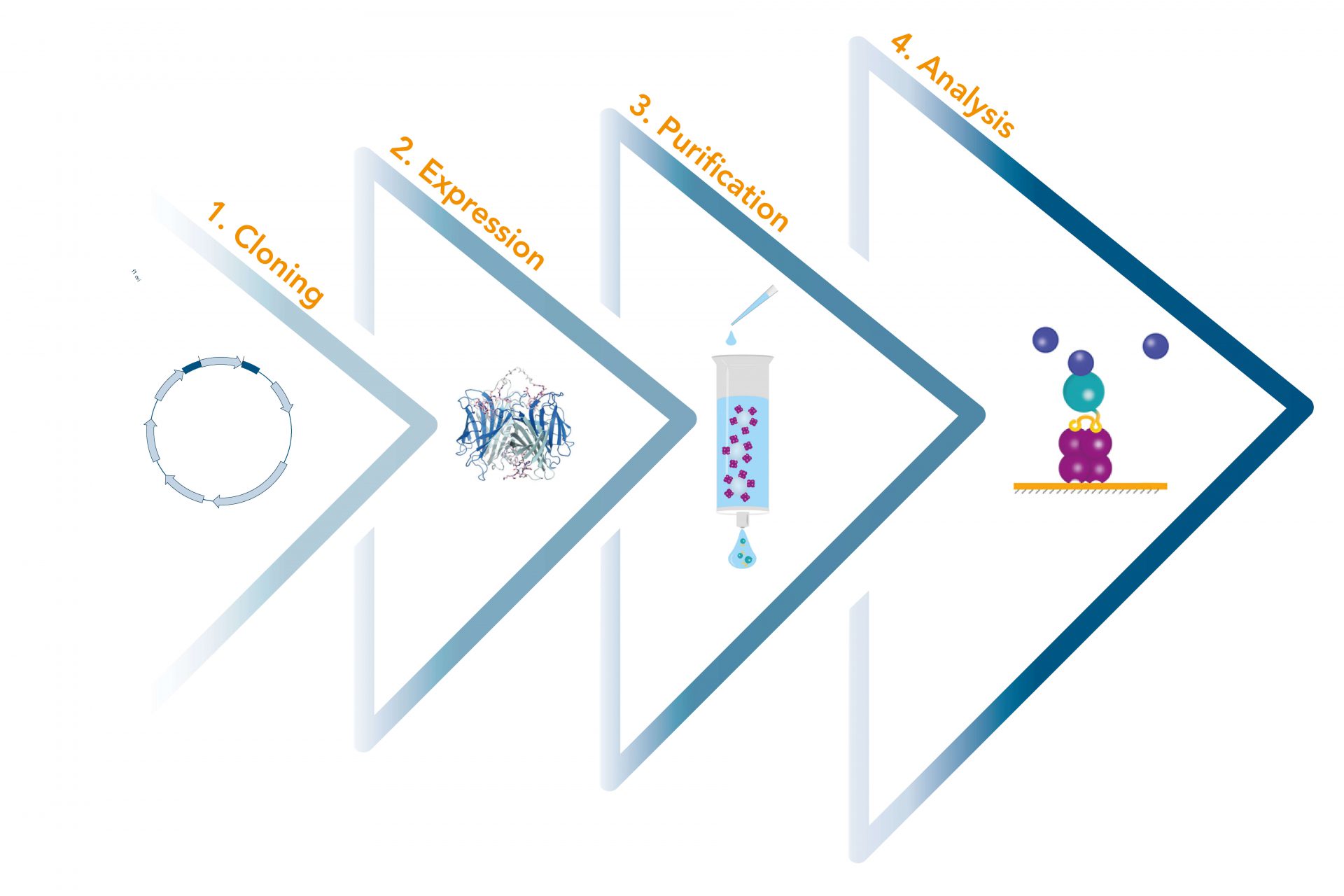
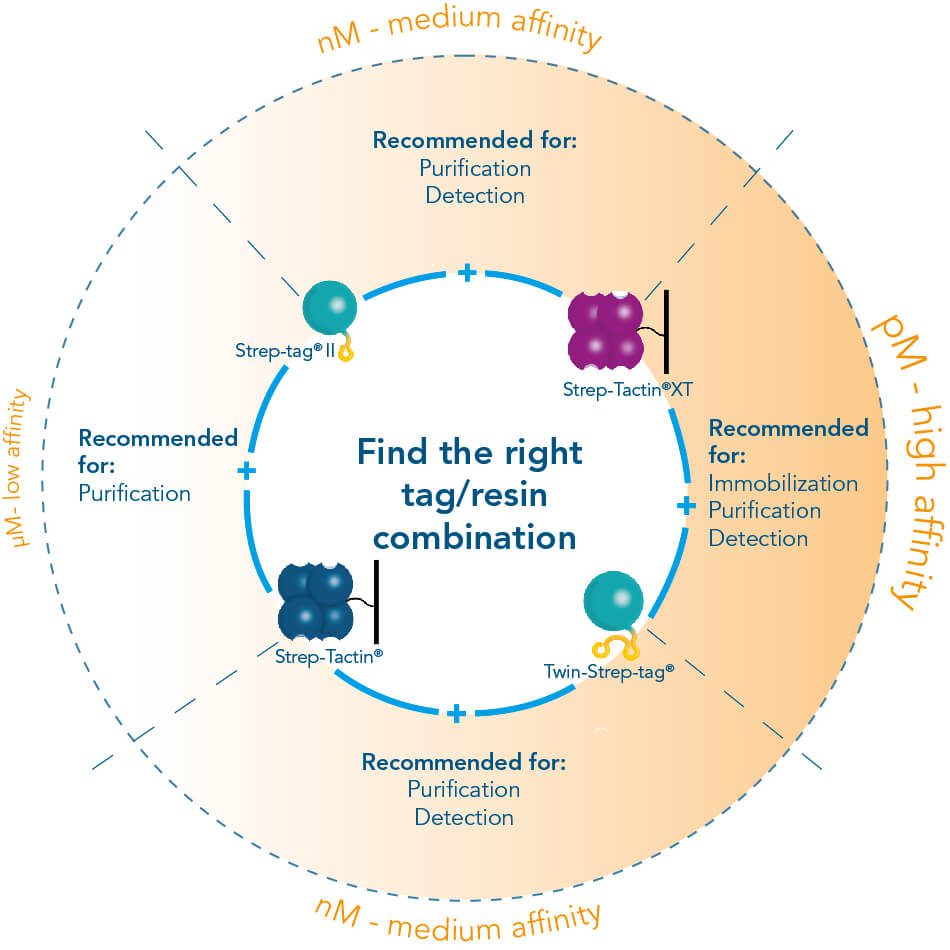
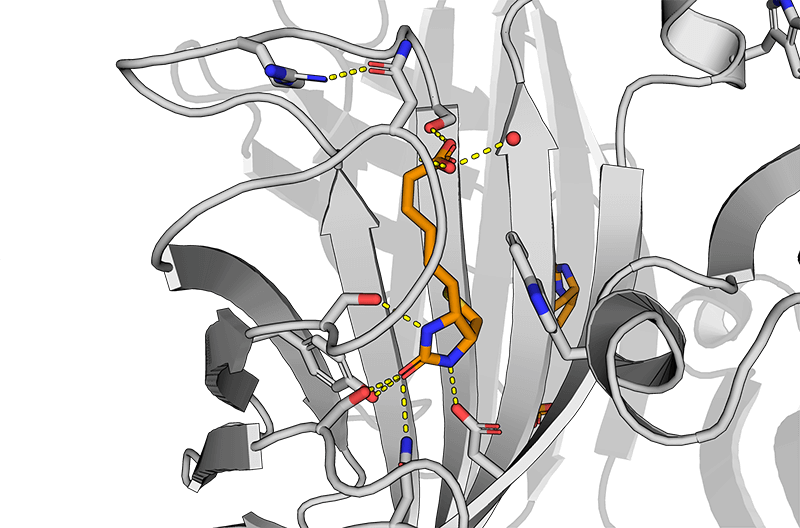
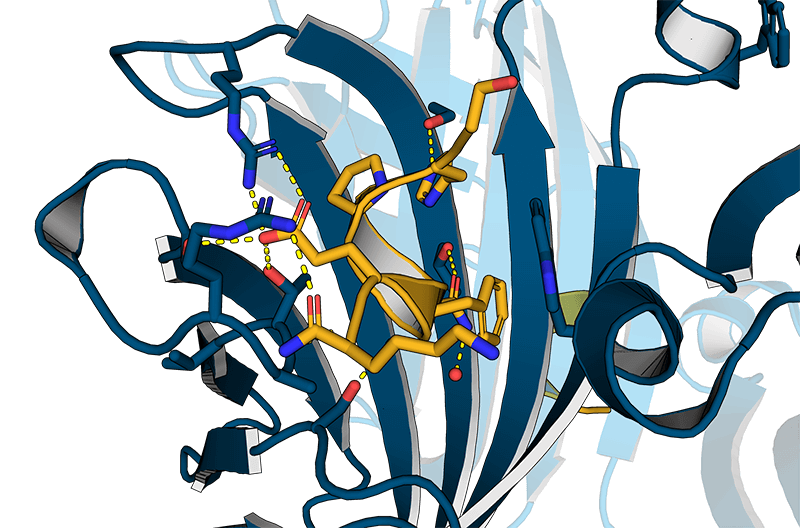
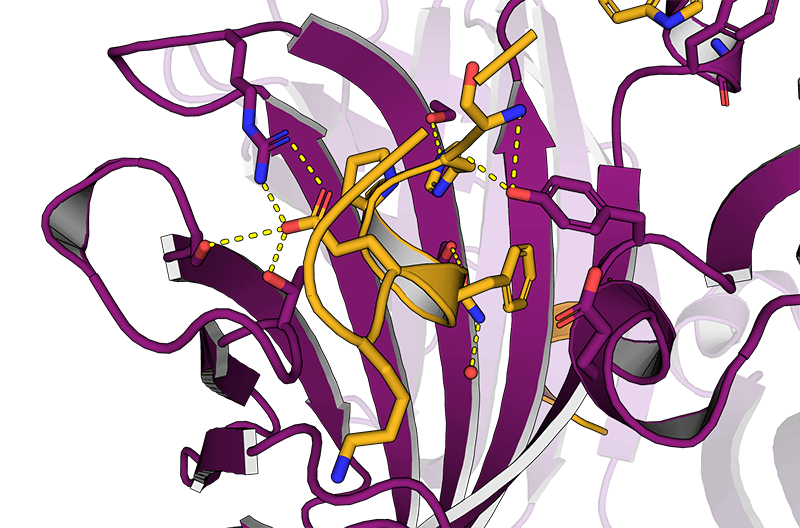
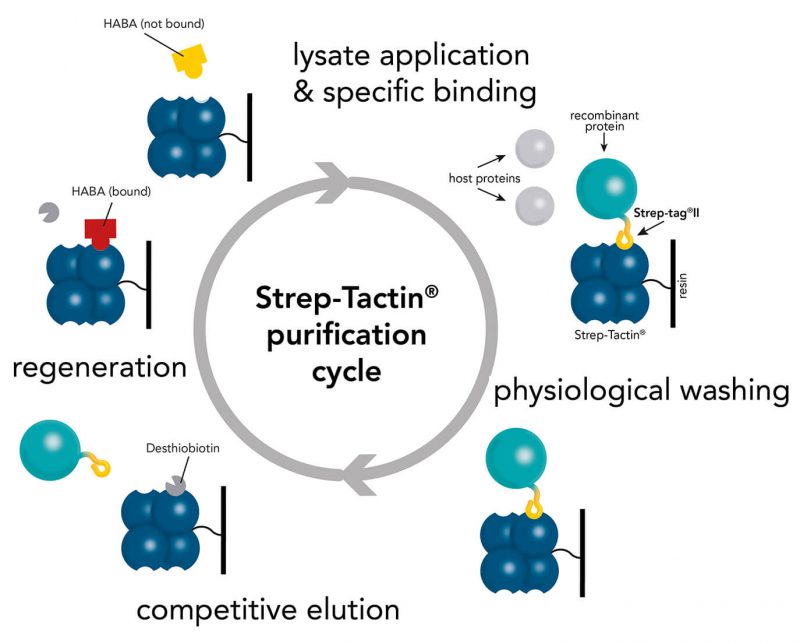
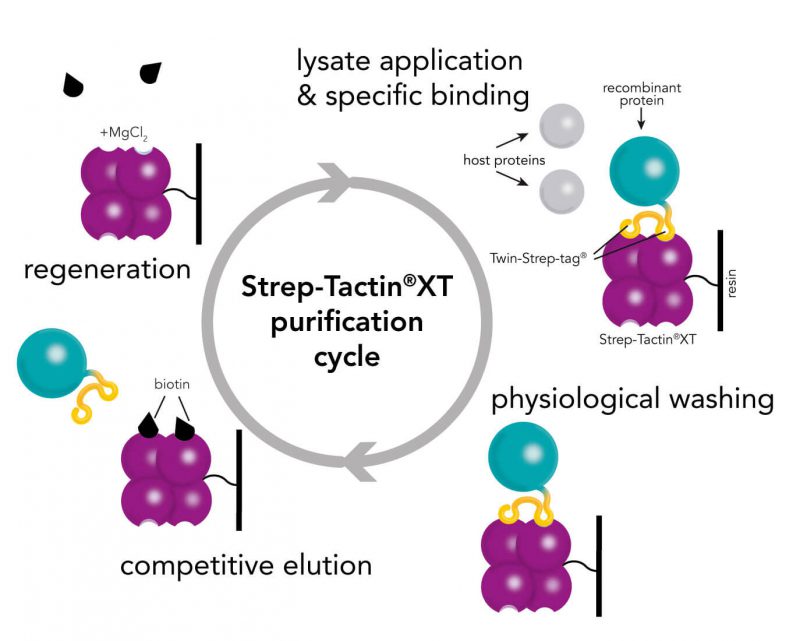
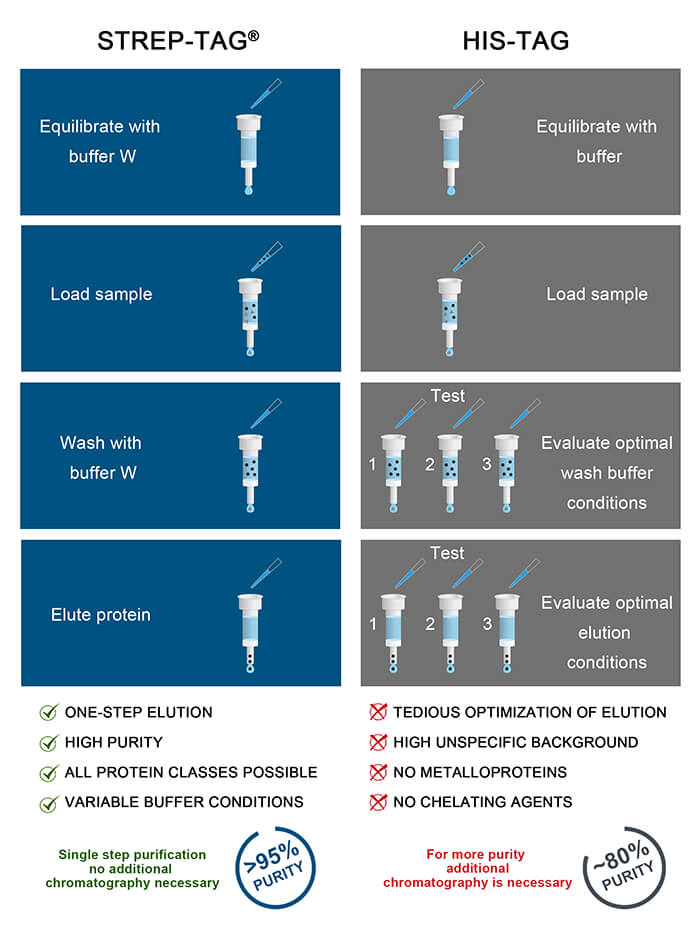
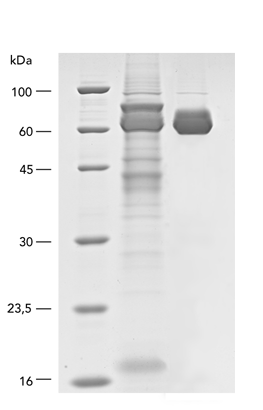
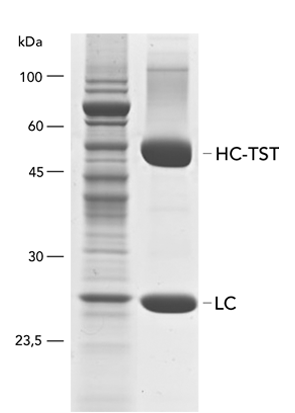
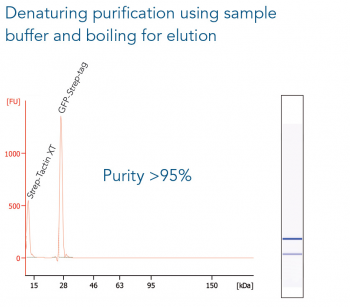
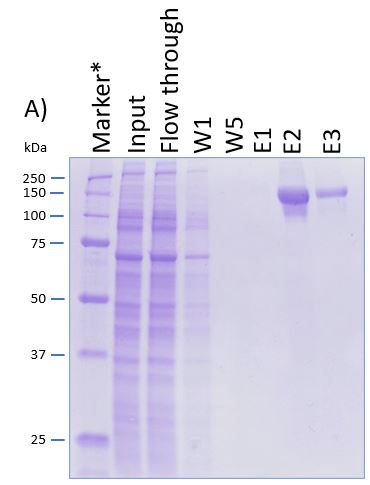
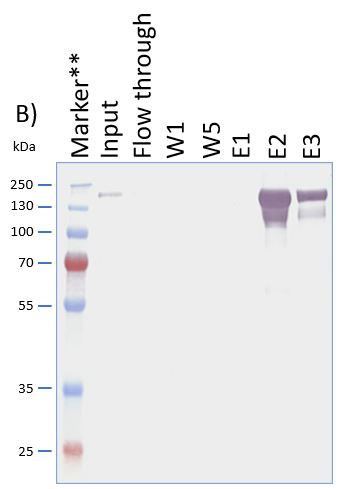
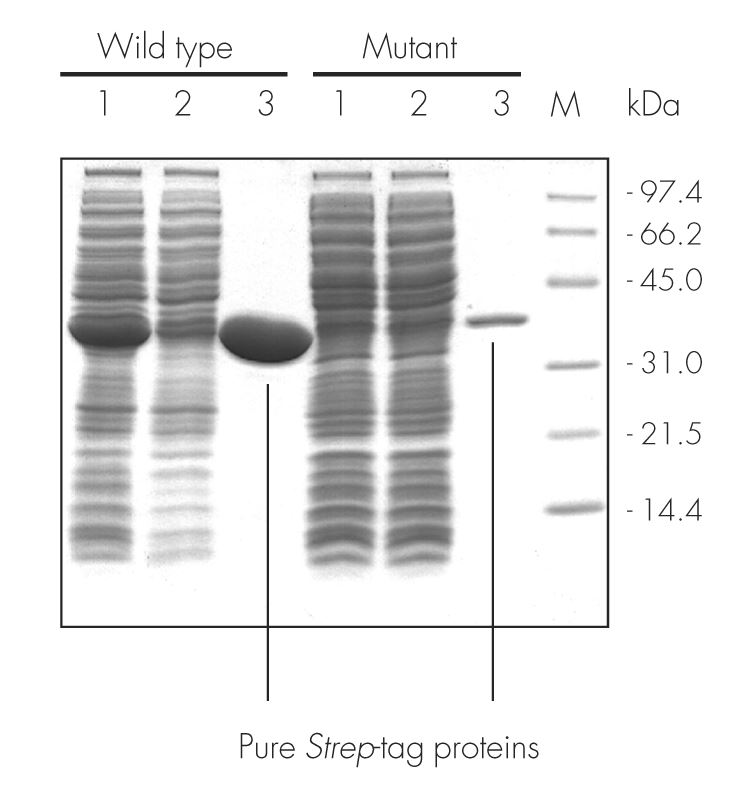
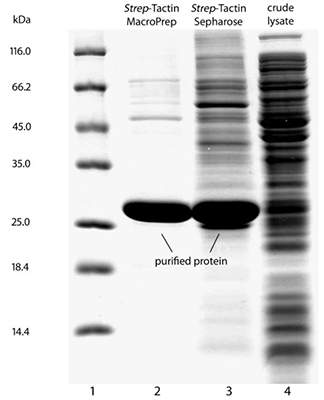
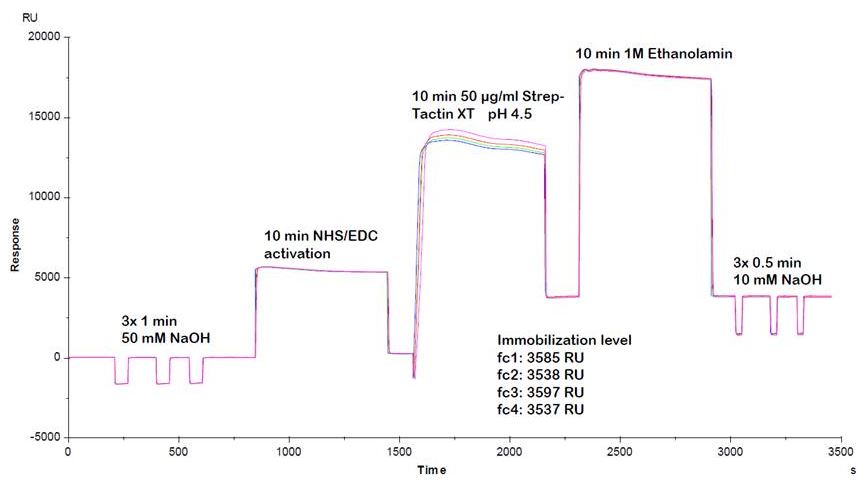
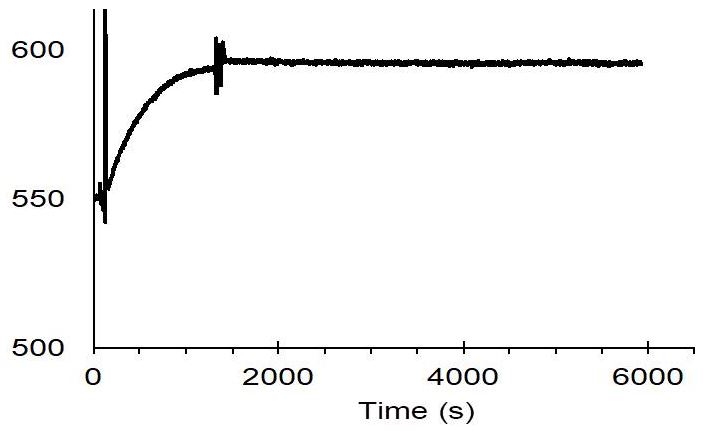
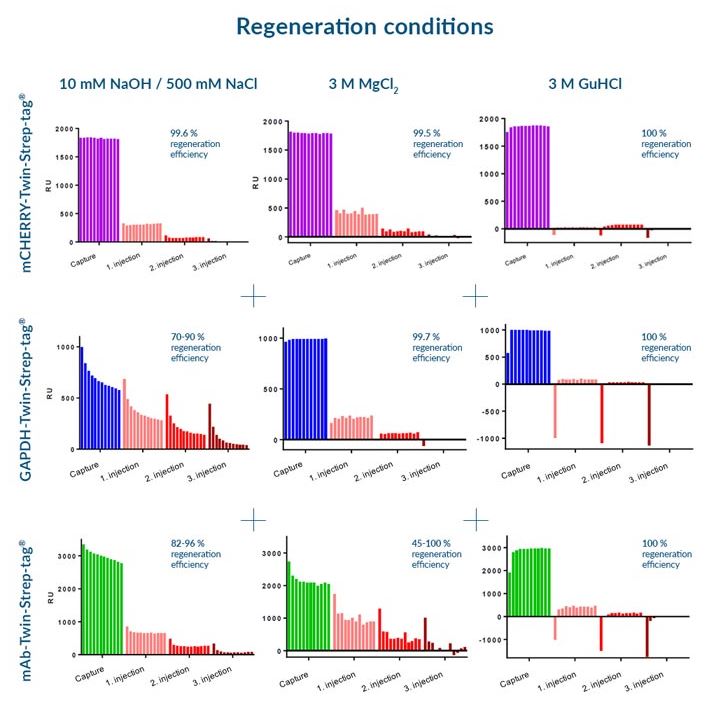
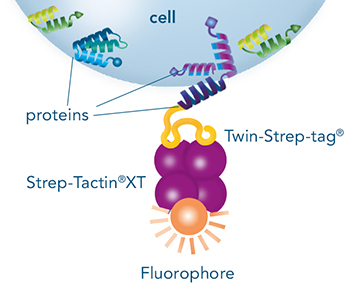
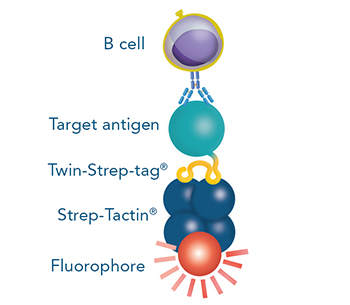
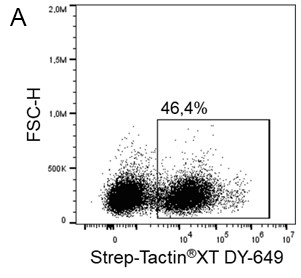
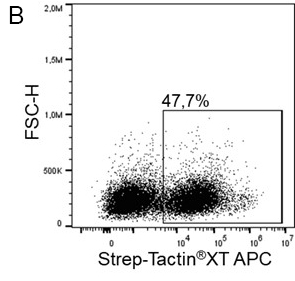
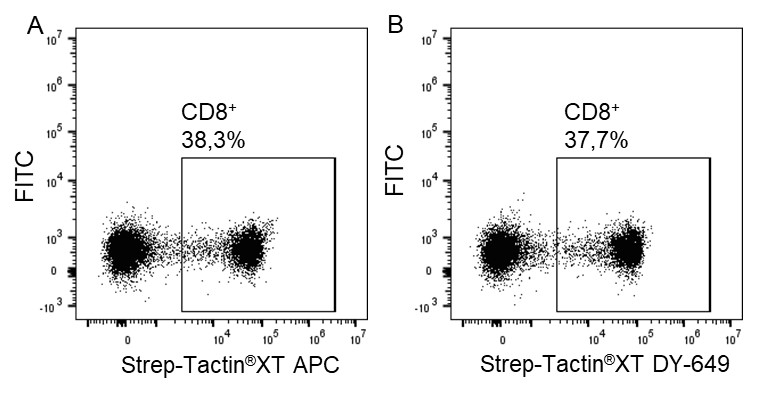
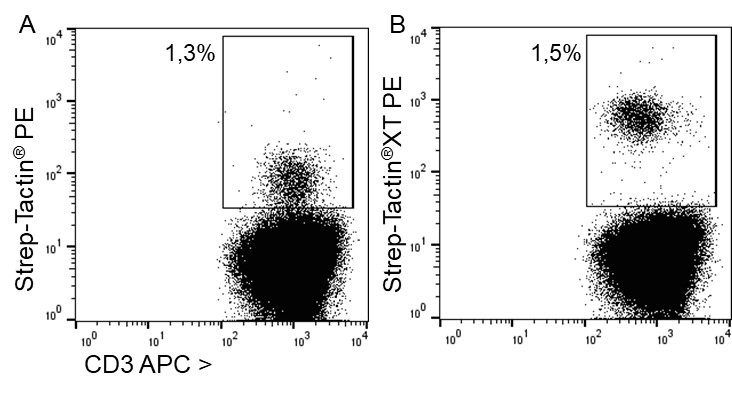
Usually, an affinity tag-free purification method is preferred. However, sometimes an affinity tag-based purification procedure can be beneficial, especially if the antibody is to be implemented in subsequent immobilization or detection. For this purpose, the application of the Strep-tag® technology is highly recommended. The antibody can be simply isolated in high purity with a resin perfectly suitable for large proteins, Strep-Tactin®XT 4Flow®. After removing biotin, the eluent, from the elution fraction via dialysis or size exclusion, the antibody can be immobilized on Strep-Tactin®XT-coated microplates or labeled with a Strep-Tactin®XT conjugate and applied for detection assays.
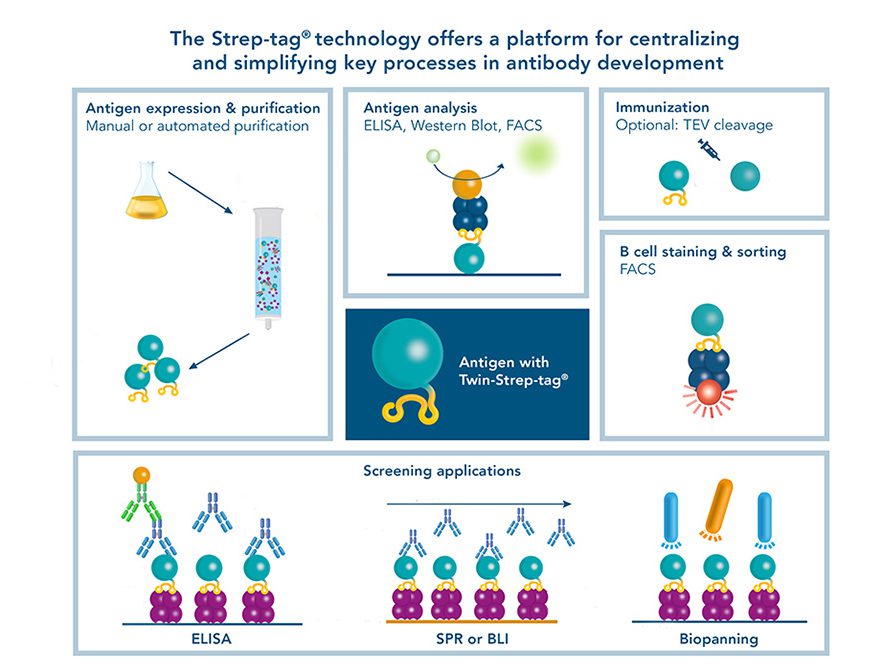
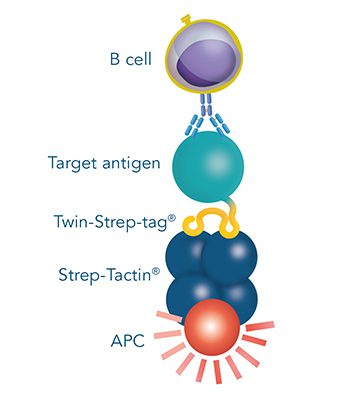
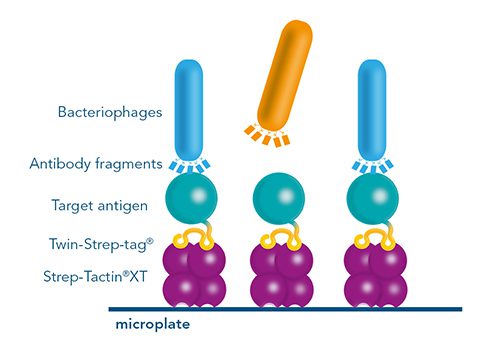
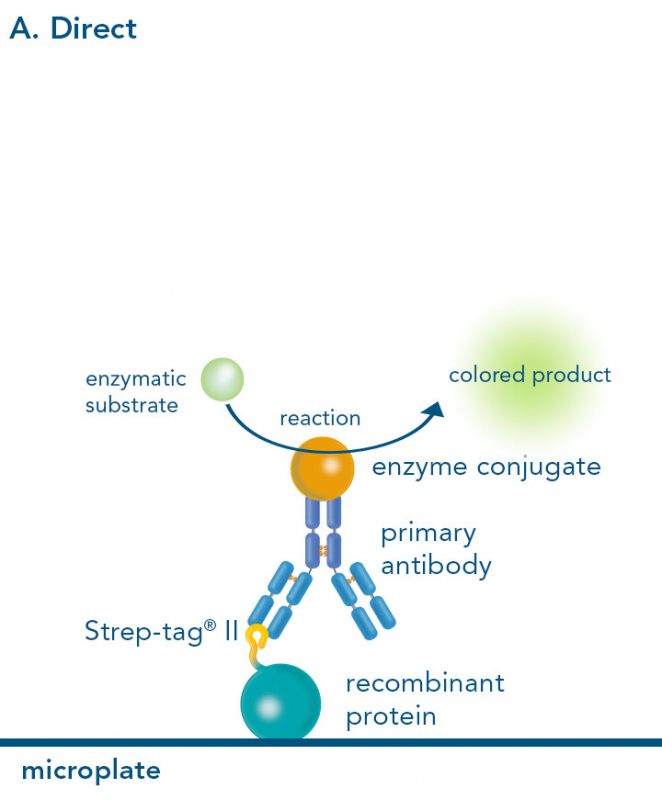
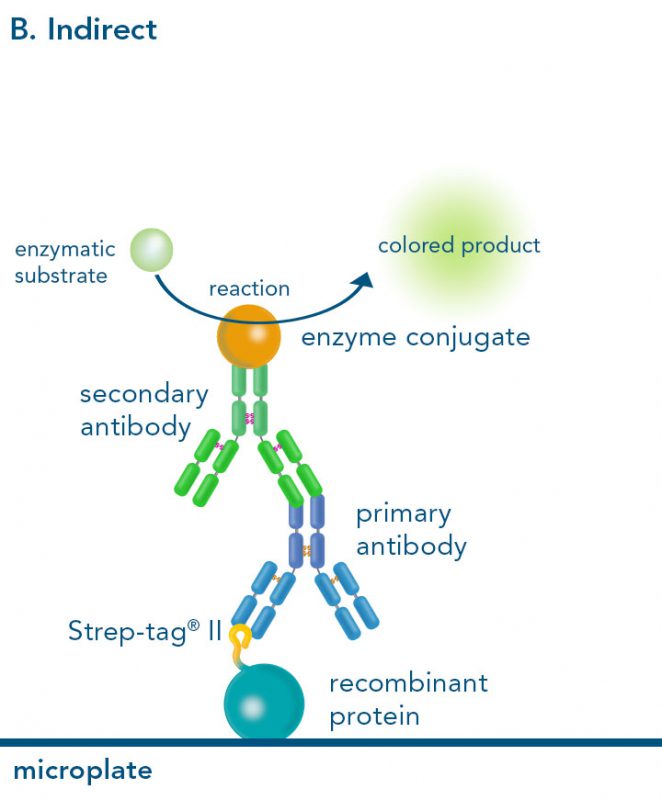
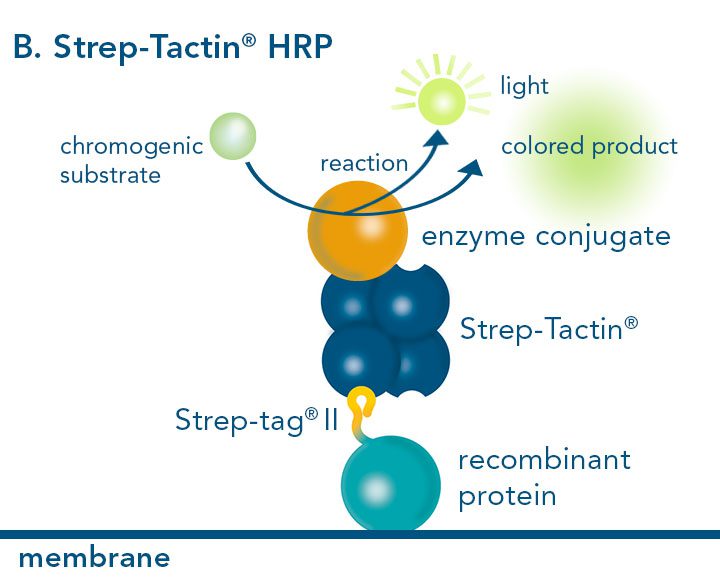
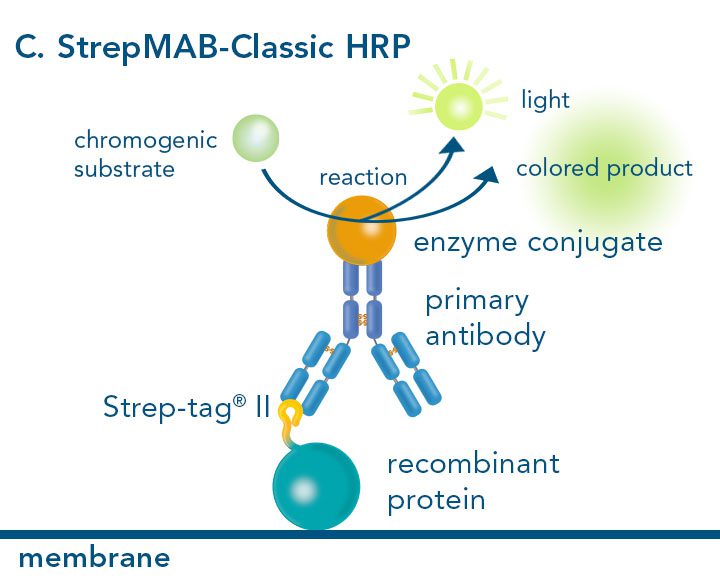
Versatility of the Strep-tag® technology for antibody purification and assay applications