Intravital microscopy has been developed to image cells and tissues in living animals. To this extent, implantation methods, as glass windows, have been designed but can induce animal distress and experiment failure and are time-consuming. To overcome those limitations, a silicon window has been developed (figure 1). This tool allows the imaging of growing tissue while being suture-free, easy to use and safe for the animal.
A silicon window for mouse intravital imaging

Figure 1: Silicon window. Scale bar: 5 mm. Credits: Hélène Vitet modified from Jacquemin et al., 2021
Why should you use a silicon window?
Living imaging is challenging because it must be adaptable to the mouse body and movements while being performant for imaging purposes. This tool is important because it widens your technical options according to the tissue you want to image. Thanks to its properties, you have now the choice between imaging a tissue underlying a bone structure like the brain, using the rigid glass window (Holtmaat et al., 2009) or superficial and growing tissue underlying the skin with the silicon window.
Indeed, this tool offers:
1- Flexibility and adaptability. The PDMS window self-adapts to the skin tension, making it the perfect tool to study growing tissues like tumors or tissues in dynamic locations like joint muscles. Even when the skin tension varies tremendously, as it does during pregnancy, the tool stays in place and does not impact biological processes like lactation.
2- Appropriate optical properties and resolution: the PDMS window is at least as good as the glass surface and offers a cellular-scale resolution (figure 2 and 3).
3- Long term monitoring: the PDMS window offers at least 3 weeks of monitoring (figure 5B).
4- Easy and fast set up: the PDMS window doesn’t need suture, glue or surgery training and its implantation over surficial tissues lasts around 5 minutes.
5- Safety: the PDMS window is safe for the animal physiology, behavior, and housing.
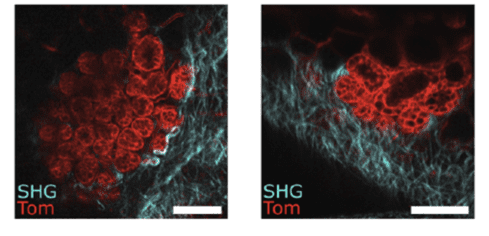
Figure 2: High-resolution IVM through the PDMS window of developing lobulo-alveolar structures in the mammary gland of a pregnant mouse, labelled with tdTomato red fluorescence (Tom). SHG shows the organization of fibrillar collagen in blue. Scale bar: 100µm. Credits: Jacquemin et al., 2021.
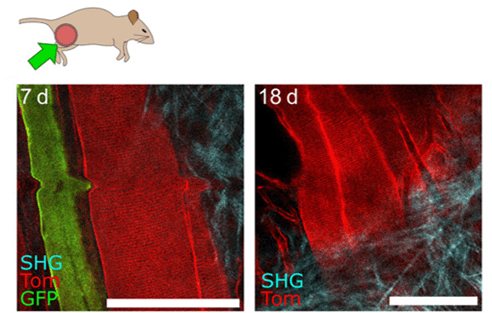
Figure 3: Representative IVM images of muscle fibers 7 (left) and 18 days (right) after implantation, showing resolution at the cellular scale and maintenance of excellent imaging quality over time. Scale bars, 100 µm. Credits: Jacquemin et al., 2021.
How does this silicon window work?
The silicon window lays on the principle of passive sealing. It is provided with a groove around its circumference with a specific angle for the skin to stay in place (point 1 figure 4) without any suture or glue. On its outside, holes were made to promote tissue self-healing, thus avoiding skin and tissue degradation (point 2, figure 4). In addition, the silicon window displays an injection port (point 3, figure 4) allowing the local injection of fluids, dyes or drugs.
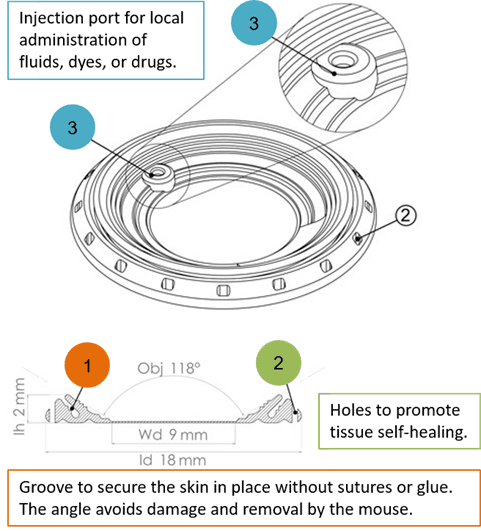
Figure 4: Three main features of the silicon window. Credits: Hélène Vitet, modified from Jacquemin et al., 2021.
Its implantation is then easy and fast since it requires only a small incision before prepositioning and fitting (Figure 5). Its ability to be folded makes this protocol easier.
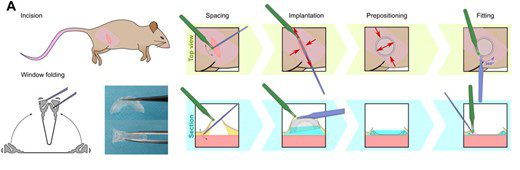
Figure 5: Steps for silicon window implantation. Credits: Jacquemin et al., 2021
What can you study with the silicon window?
The silicon window is the perfect tool if you want to image growing and/or soft tissues. This is for example the case for studies on regeneration (figure 6), stem cells, tumors, development, or pharmacokinetics where in vivo multiple-scale imaging overtime is required.
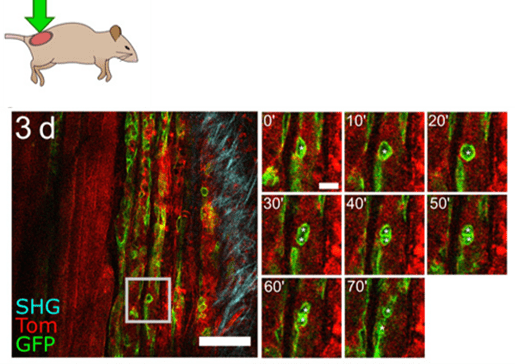
Figure 6: IVM of thigh muscle regeneration through the PDMS imaging window 3 days after cardiotoxin-induced muscle injury in adult mice. Time-lapse imaging shows muscle stem cell division and migration (white asterisks) over 70 min. Scale bar, 100 µm. Credits: Jacquemin et al., 2021
In a nutshell
This new intravital microscopy device gives you the opportunity to image both cells and tissues in an integrated way, especially for superficial tissues in dynamic locations. It recapitulates many features that make it safer, easier to use and more adaptable than the window based on a glass coverslip fixed within a rigid frame.
References:
Holtmaat, A., Bonhoeffer, T., Chow, D. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc 4, 1128–1144 (2009). https://doi.org/10.1038/nprot.2009.89
Intravital Microscopy by Guillaume Jacquemin
