Assay Setup: Sandwich ELISA for Allergy
Sandwich ELISA is the most commonly-used ELISA format, which uses matched antibody pairs for capture and detection to enable specificity and sensitivity for the analyte of interest from complex mixtures such as biological samples. The Sandwich format enables superior specificity compared to either a direct or indirect ELISA because there are two distinct analyte-binding antibodies. Each of the antibodies are able to recognize a different antigenic epitope, preventing competition.
Set up
The sandwich format may be performed directly or indirectly. It uses an analyte-specific capture antibody which is bound directly to microplate wells and captures the analyte from the sample. The sample is added to the well, and the plate-bound antibody captures the analyte from this sample. A second analyte-specific “primary” antibody which is also specific to the analyte, is then added. This antibody may or may not be labeled with a reporter molecule, depending on whether it is a direct or indirect sandwich. If the “Primary antibody” is unlabeled, then a third “Detection” or “Secondary” antibody is added. Conjugated to a reporter enzyme or a fluorescent probe, it acts to amplify the achievable signal.
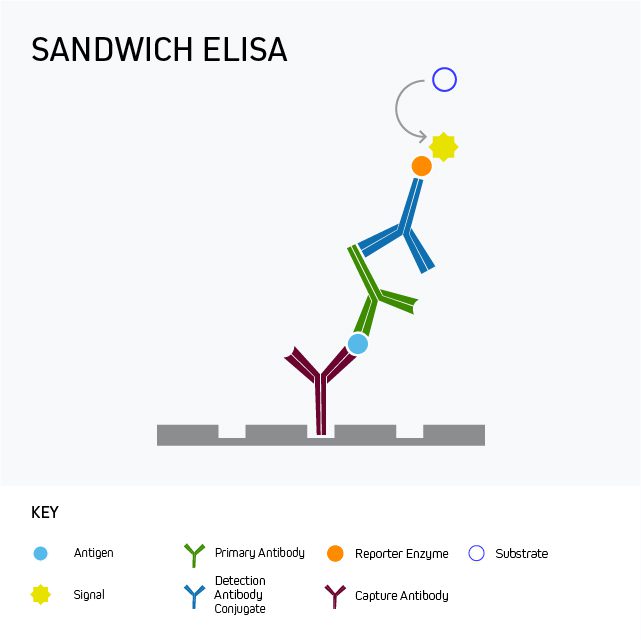
Figure 1: Sandwich ELISA benefits from superior specificity compared to either a direct or indirect ELISA because two distinct analyte-binding antibodies (each recognizing a different antigenic epitope) are used. It may also provide greater sensitivity, and even low-abundance antigens can be detected within complex samples such as serum.
The indirect sandwich ELISA:
Polyclonal or Monoclonal?
The clonality of the antibodies chosen can impact the performance of the assay. Monoclonals can offer consistent lot-to-lot performance and guaranteed long-term supply, whereas polyclonals can increase the likelihood of target detection and improve sensitivity.
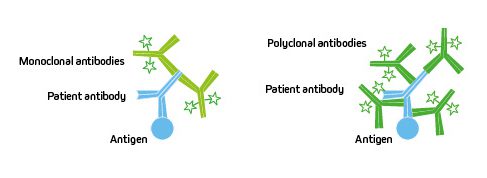
Figure 2: Monoclonal antibodies are comprised of a one immunoglobulin clone which detect a single antigenic epitope, making the antibody very specific but limiting it to one site. Polyclonal antibodies are made up from a heterogenous population of immunoglobulins which detect a variety of epitopes and can bind multiple sites across the target.
Some assays may incorporate a monoclonal antibody for antigen capture and a polyclonal antibody for detection, or they may use a pair of monoclonal antibodies. If the sandwich ELISA relies on using matched monoclonal antibody pairs it is important that they have been rigorously validated for the ELISA application.
Validate antibodies
If using monoclonal antibodies it is critical to prevent the two antibodies from competing with one another to bind the target epitope. Performing control experiments ensures that the analyte-specific antibodies can detect the antigen under the conditions of the ELISA, where proteins may be in their native forms rather than those used to confirm reactivity by Western blot. Competition assays may be used to confirm that two different epitopes are targeted by the antibody pairs.
If using polyclonal antibodies, choose min x to reduce off-target signal
Experiments may need a combination of monoclonal and polyclonal antibodies, which will require the experimental design to consider the impact of host and sample species, and any blocking reagents that can potentially cross-react, generating unwanted background signal by introducing non-specific binding. Choosing an antibody from Jackson ImmunoResearch that is minimally cross-reactive against the sample species, and other antibodies’ host species is desirable to minimize non-specific background.
Example 1: Indirect Sandwich ELISA with Human serum sample
| Antibody identity | Clonality | Specificity | Host | Min x |
| Capture | Polyclonal | Antigen A | Rabbit | Hu, Dnk, Bov |
| Primary | Monoclonal | Antigen A | Rabbit | |
| Detection | Polyclonal | Rabbit | Donkey | Hu, Dnk, Bov |
| Blocking | BSA |
Table 1: Setting up a 3-step indirect sandwich ELISA using a combination of polyclonal and monoclonal antibodies
Orientation
Although the antibody pairs may be interchangeable as the capture or primary antibody, the orientation of the antibody can impact the sensitivity and specificity of the assay. You may wish to compare how your antibodies perform in either role. We typically choose to use a min X antibody as the primary/detection antibody.
Another aspect of orientation to consider is which part or region of the antibody specificity is directed towards. Fc and F(ab’)2 specific antibodies are available and can be implemented if targeting of particular epitopes are required.
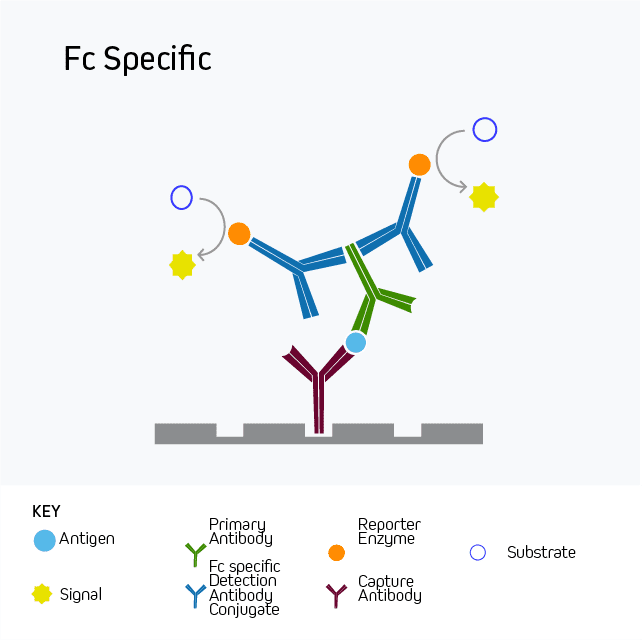
Figure 3: Fc and F(ab’)2 specific antibodies can be used to “orient” an assay towards a particular region of the partnered antibody in cases where particular epitopes may need to be preferentially bound in a particular order or direction.
Blocking
Aft the capture antibody is applied the plate is typically blocked using serum from the same host species as the conjugated detection antibody, or with IgG and protease-free Bovine serum albumin (BSA).
The sample
The nature of the sample can add extra consideration to the assay. Matrix effects are the artifacts that are caused by the components of the sample, such as lipids, sugars and proteins which can change the way an ELISA performs. Dilution of the sample can sometimes reduce the impact of these matrix effects and improve the signal-to-noise ratio.
Detection of IgE from plasma using a direct sandwich ELISA
Example 2: Human serum
| Antibody | Clonality | Specificity | Species | Conjugate |
| Capture | Monoclonal | Human IgE | Mouse | |
| Detection | Monoclonal | Human IgE | Mouse | Alkaline Phosphatase |
| Blocking | BSA |
Table 2: A 2-Step direct sandwich ELISA set-up
Jackson ImmunoResearch Anti-Human IgE offers excellent sensitivity when used in a sandwich ELISA with its capture partner antibody.
In this format, use our unconjugated Anti-Human IgE ( clone number 10A10 209-002-244) as your coating antibody before blocking with Bovine Serum Albumin (IgG-Free, Protease-Free 001-000-161). Following washing, incubate with your diluted sample.
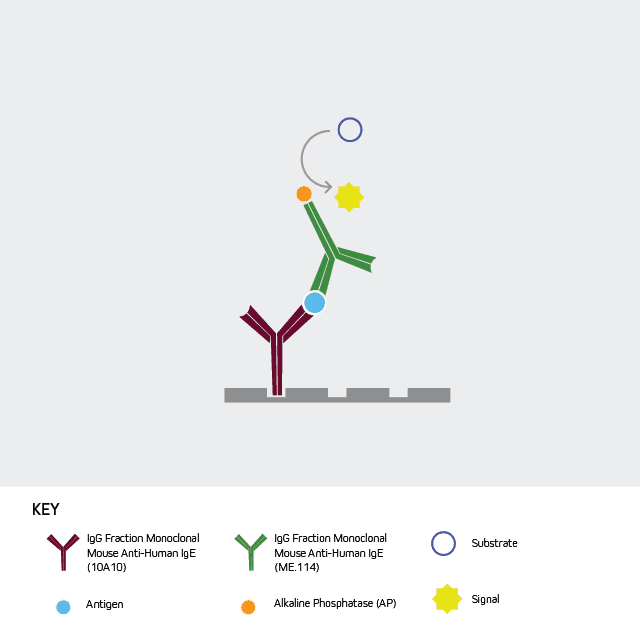
Figure 4: Direct Sanwich ELISA. Unconjugated Anti-Human IgE ( clone number 10A10 209-002-244) is used as the capture antibody and a conjugated Anti-Human IgE antibody, such as Alkaline Phosphatase Anti-Human IgE (209-052-241) is used as the detection antibody.
For the detection step, use a conjugated Anti-Human IgE antibody, such as Alkaline Phosphatase Anti-Human IgE (209-052-241) or Peroxidase Anti-Human IgE (209-032-241), followed by the appropriate substrate or visualization technique.
Figure 5 illustrates the application of the direct sandwich assay to validate the quantification of total IgE from patient samples using unconjugated Anti-Human IgE in partnership with an Alkaline Phosphatase conjugated detection antibody. Total IgE (kIU/l) in patient samples used in this experiment were previously measured by the Roche Cobas Total IgE assay. These results are displayed on the x-axis of Figure 5.
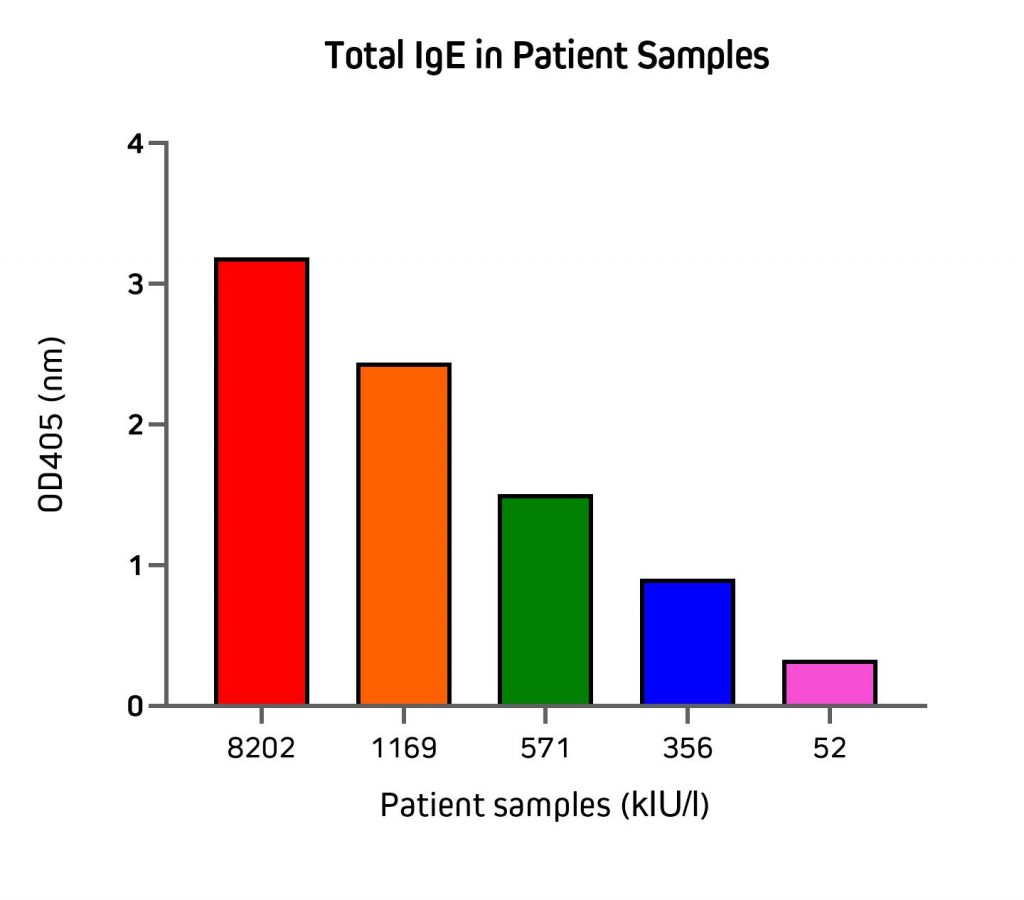
Figure 5: Detection of total IgE from human serum and plasma samples by direct sandwich ELISA. Plate was coated with unconjugated Anti-Human IgE (clone 10A10 209-002-244) antibody at 100μl/well at 2μg/ml in coating buffer overnight at 4oC. The plate was blocked with 300μl/well of 1% BSA (001-000-161) in Phosphate buffered saline, 0.05% Tween® (PBS/T) for 1.5 hrs. Incubate with 100μl/well of plasma/serum samples diluted 1:80 in PBS/T for 1hr. Detection antibody, Jackson ImmunoResearch Alkaline Phosphatase Anti-Human IgE (209-055-241), was diluted at 1:10,000 in PBS/T and incubated at 100μl/well for 1 hr. 100μl/well of freshly prepared DEA substrate was incubated on the plate for 30 min and read at 405nm.
