Immunoglossary
Affinity
The strength of a binding interaction between the antigen-binding site (paratope) on an antibody and the epitope on an antigen. Also known as binding affinity.
Antigen
Any material which elicits an immune response (e.g., a bacterial toxin or viral protein).
Avidity
The overall strength of the antibody-antigen complex, resulting from binding affinity, antibody valency, and the structural arrangement of the complex. Also known as functional affinity.
Complementary Determining Regions (CDRs)
Short sequences of amino acids found on the antibody variable region that collectively form the paratope. An IgG antibody has 12 CDRs, with two Fab Fragments comprising three CDRs on each of the heavy and light chains. *A heavy chain-only antibody, found in species from the Camelid family, has 6 CDRs, with two VHH Fragments comprising three CDRs on each of the heavy chains.
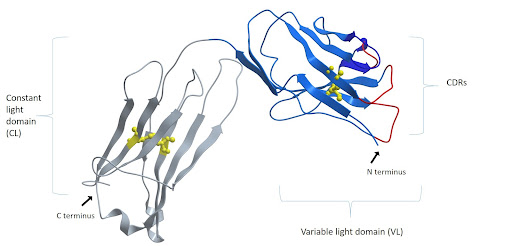
Figure 1: The light chain consists of a variable and a constant domain. Each domain is made of two layers of polypeptide chains, forming two tightly packed anti-parallel β-sheets. One comprised of four β-strands and another of three, each layer interconnected by an intramolecular disulfide bond (shown in yellow). The folding of the layers results in looped stretches which make up the 3 CDRs shown in red. Image of PDB code 1igt generated with Molsoft ICM Pro. (https://www.mdpi.com/2073-4468/8/4/55/htm) .
Constant domains
Distinct sections of the antibody constant region. Each light chain contains one constant domain (CL), while the heavy chain of IgA, IgG, and IgD has three constant domains (CH1, CH2, and CH3, where CH1 is nearest the antibody variable region ). Both IgM and IgE contain four constant domains (CH1-CH4).
Constant region
Part of the antibody molecule that is identical between all antibodies of the same isotype, comprised of one or more constant domains.
Cross adsorption
An affinity purification process for removing antibodies that bind to off-target species (e.g., for ensuring a goat anti-mouse secondary antibody binds only to primary antibodies raised in mouse).
Epitope
The region on an antigen that is recognized by an antibody.
Fab Fragment
Part of the antibody molecule produced using papain to cleave above the hinge region, comprising one constant domain and one variable domain of each of a heavy and light chain. Also known as the Fragment antigen-binding.
F(ab’)2 Fragment
Part of the antibody molecule produced using pepsin to cleave below the hinge region, comprising two Fab Fragments joined by disulfide bonds.
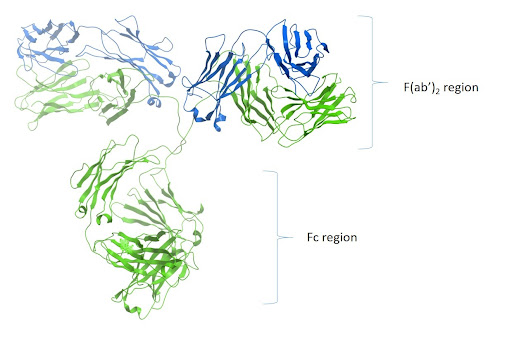
Figure 2:. Ribbon image of PDB code 1igt generated with Molsoft ICM Pro.
Figure 3: F(ab’)2 Fragments are produced by pepsin digestion which cleaves the Fragment from the Fc region retaining a bridging disulfide bond interconnecting two Fab arms making the molecule bivalent.
Fc Fragment
Part of the antibody molecule produced using papain to cleave above the hinge region, comprising the CH2 and CH3 domains in the case of IgG. Mediates antibody function through binding to Fc receptors (FcRs) on effector cells. Also known as the Fragment crystallizable (Fc) region for its ability to crystallize.
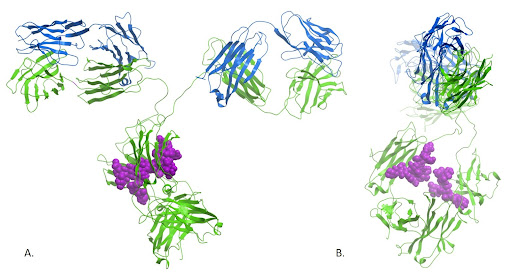
Figure 4: The crystal structure of the immunoglobulin reveals the close proximity of the N-glycosylations present at Asn297. These sugars are thought not only to contribute to the structural conformation and stability of the Fc domain, but also mediate receptor binding through carbohydrate-carbohydrate interactions. Ribbon image of PDB code 1igt generated with Molsoft ICM Pro.
Framework regions
Structural components of the variable domain, found between the complementary determining regions (CDRs).
Globulins
Serum proteins that are categorized loosely by their electrophoretic mobility as alpha globulins that inhibit proteases, beta globulins that exist as various enzymes, and gamma globulins that mainly comprise immunoglobulins (antibodies).
Heavy chain
The larger of the two types of polypeptide chain that make up an antibody molecule, comprising a constant region with three (IgA, IgG, and IgD) or four (IgM and IgE) constant domains and a variable region.
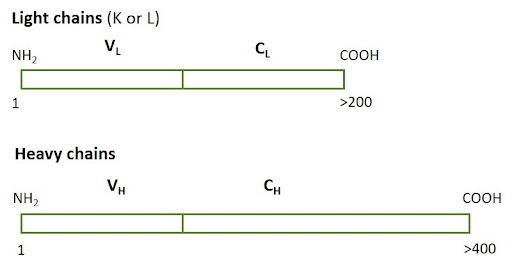
Figure 5: Heavy chains are typically larger than light chains, consisting of more than one constant domain. Light chains can be either K (Kappa) or Lambda (L).
Heavy chain-only
A heavy chain-only antibody, found in species from the Camelid family (IgG2 and IgG3), has 6 CDRs, with two VHH Fragments comprising three CDRs on each of the heavy chains and lack the CH1 domain.
Idiotype
The antigenic determinant created by the hypervariable regions (paratope) of the individual antibodies. A property of antigen recognition; antibodies sharing the same idiotype all detect the same epitope of an antigen.
Immunogen
A term commonly used to describe material that is deliberately introduced into an individual to elicit an immune response. See antigen.
Immunoglobulin
Another term for an antibody.
Immunoglobulin class (Isotype)
Classification based on the antibody heavy chain; IgA antibodies have α-chains, IgDs have δ-chains, IgEs have ε-chains, IgGs have γ-chains and IgMs have μ-chains. Also known as isotype.
Immunoglobulin subtype
Sub-classification of immunoglobulin class (isotype) based on differences in the heavy chain. Human IgG antibodies are divided into four subclasses (IgG1, IgG2, IgG3, and IgG4, listed according to decreasing abundance in the serum), while rabbits have only one IgG subclass and mice have five.
Light chain
The smaller of the two types of polypeptide chain that make up an antibody molecule, comprising a constant region and a variable region. In mammals, there are two classes of light chain; the kappa (κ) chain that is more abundant, and the lambda (λ) chain that has four subtypes.
Monoclonal antibody
A type of antibody traditionally produced by fusing a B cell with an immortal myeloma to form a hybridoma, but more recently generated via recombinant methods. Monoclonal antibody preparations consist of identical antibody molecules that all recognize the same epitope.
Paratope
The region on an antibody that binds the epitope; comprised of the CDR sequences.
Polyclonal antibody
A type of antibody typically collected from the sera of an animal. Polyclonal antibody preparations consist of non-identical antibody molecules that recognize different epitopes.
Primary antibody
An antibody raised against an antigen of interest (e.g., a cytoskeletal protein such as actin).
ScFv
A single chain variable fragment; recombinant protein consisting of heavy and light chain variable regions joined by a linker.
Secondary antibody
A species-specific antibody that is conjugated to a detection moiety (e.g., an enzyme or a fluorophore) to allow monitoring of primary antibody binding to the target.
Selectivity
A measure of how well an antibody binds its antigen in a heterogeneous mixture; selective antibodies are preferred for research to avoid unwanted cross-reactivities.
Specificity
A measure of how well an antibody recognizes and binds to its intended target; specific antibodies are preferred for research to avoid unwanted cross-reactivities.
Target species
The species a secondary antibody is directed against. (e.g., the target species of a goat anti-mouse antibody is mouse).
Valency
The number of antigen-binding sites found on an antibody; IgG antibodies are bivalent, while pentameric IgMs have ten antigen-binding sites. Fab or VHH Fragments are monovalent.
Variable region
Part of the antibody molecule that dictates which antigen is recognized. Each antibody heavy and light chain has one variable region. Also known as the Fv region.
VHH Fragments
Part of the Camelid IgG2 or IgG3 antibody molecule produced using papain to cleave above the hinge region, comprising one variable domain of a heavy chain. Also known as the nanobody.
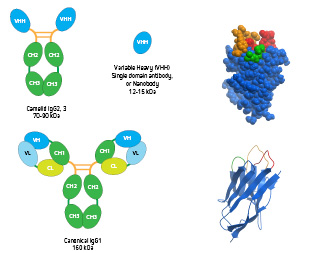
Figure 6: Conventional IgG, heavy chain-only IgG and VHH domain antibody. Inset: Space filling and ribbon diagram of the VHH domain antibody (PDB 1MEL) showing the 3 CDR loops, with the longer CDR3 loop shown in orange.
References:
Chiu ML, Goulet DR, Teplyakov A, Gilliland GL. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies. 2019; 8(4):55. https://doi.org/10.3390/antib8040055
