The attachment of a reporter group (label) is often required for the detection and biochemical/cellular characterization of proteins and its binding partners.
Different labeling techniques & types of label such as fluorophores, biotin and luminescent dyes are available for non-radioactive protein labeling, both random and site-directed (N- or C-terminal).
Choose the appropriate labeling strategy (labeling technique & type of label) that fits your final application based on your initial protein source (Tab. 1).
Table 1: Protein Labeling Selection Matrix
| Type of Label | |||||||
| Fluorophore | Biotin | Desthiobiotin | CLICK functionality | ||||
| Protein Source | Purified protein | Amine Labeling | Amine Labeling | Amine Labeling → Click Chemistry | random | Labeling Position | |
| Thiol Labeling | Thiol Labeling → Click Chemistry | ||||||
| Proliferating cells (Nascent proteins) | Amino acid-based Metabolic Labeling | ||||||
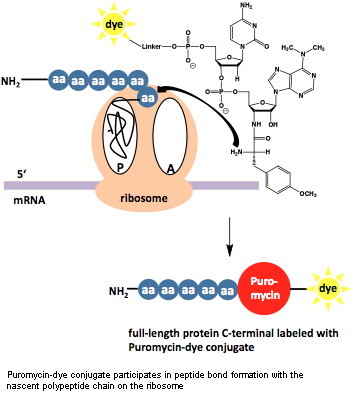
| Puromycin-based Metabolic Labeling | site directed | ||||||
| ATP dependent Lysine-based Labeling | ATP dependent Lysine-based Labeling | ||||||
| Protein encoding DNA sequence (Cloning & cell-free expression required) | dC-Puromycin-based Co-translational Labeling | dC-Puromycin-based Co-translational Labeling | |||||