Figure 2. The application of base editing technology (using CBE as an example).
Reversing disease-causative point mutations
Single nucleotide polymorphisms (SNPs) are the most common type of mutation associated with human diseases, with 70% of these mutations potentially correctable through base editing. This sheds light on a new pathway to rectify genetic defects [5].
β-thalassemia is a blood genetic disorder caused by mutations in the HBB gene. The HBB-28 (A>G) mutation is one of the top 3 common mutations observed in the patient population in China and Southeast Asia. It has been reported that using base editors (BEs) to correct the HBB-28 (A>G) mutation in patient-derived primary cells were able to achieve an editing efficiency of over 20% [6].
Base editing can also be used to reverse pathogenic mutations in patient-derived induced pluripotent stem cells (iPSCs), achieving an editing efficiency of over 80% These iPSCs can be applied in disease mechanism studies, drug screening, and cell therapy development [7].
Introducing stop codons for Gene Knockout
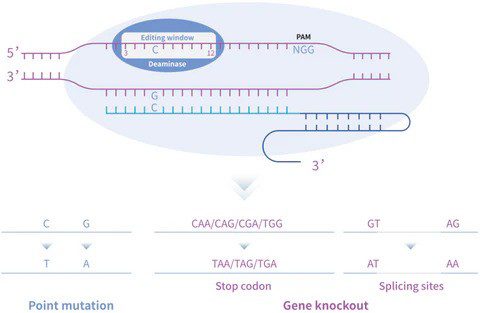
Cytosine base editors (CBEs) can convert C•G base pairs into T•A, introducing a premature stop codon to disrupt gene expression. Substituting cytosines in coding sequences to thymine can generate stop codons like TAA, TAG, or TGA. This method has been reported to have similar efficiency to Cas9-mediated gene knockouts [8].
Disrupting RNA splicing sites for Gene Knockout
Gene knockout can also be achieved by disrupting mRNA splicing sites such as splice donor (SD) sites and, potentially, more advantageous over introducing stop codons directly, as altering the splicing site can avoid issues with stop codon reading under stress conditions. Editing RNA splicing sites changes gene processing at the mRNA level and therefore, blocks protein translation.
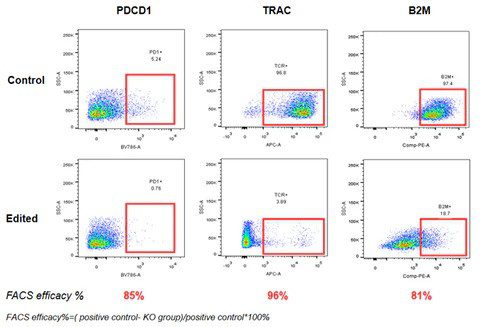
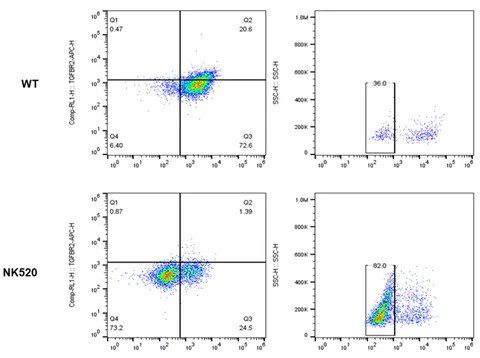
An example of applying base editing technology in gene knockout includes the knockout of TRAC, B2M, & PD1 genes to create allogenic CAR-T cells. Delivering base editors in the form of ribonucleoproteins (RNPs) via electroporation for triple gene knockouts achieved over 90% gene knockout efficiency in primary T cells [9].
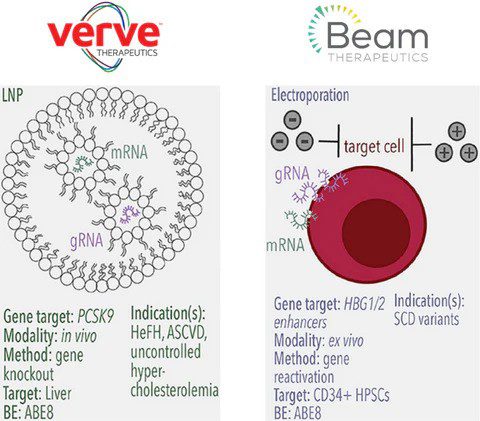
The VERVE-101 leverages an adenine base editor to alter the PCSK9 mRNA splicing site, leading to aberrant splicing and nonsense-mediated decay, thereby reducing PCSK9 protein levels. These examples demonstrate the versatility and potential of base editing technology in creating precise genetic modifications for therapeutic purposes, offering hope for treating a wide range of genetic disorders.
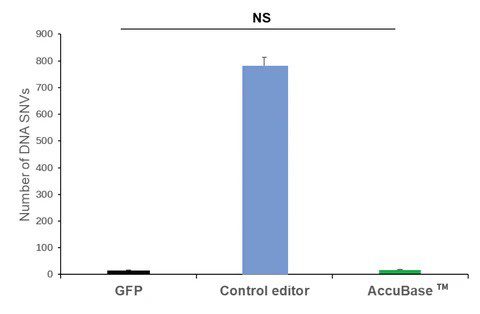
The AccuBase™ “near-zero off-target” base editing tools
After a decade of competition, gene editing technology has developed into the 2.0 phase represented by base editors [10]. The CBE base editing technology AccubaseTM, independently developed and owned by Base Therapeutics, has been validated for its high-efficiency and precision gene editing capabilities across various cells and applications. In 2023, Base Therapeutics joined forces with KACTUS in a strategic partnership for the global production and sales of the GMP-grade base editor AccuBaseTM. Today, the AccuBaseTM products are globally available in stock, supporting CGT drug development.
Key achievements with AccuBase™ include:
High-efficiency gene knockout in activated primary T cells using AccuBase™ RNP, achieving over 80% knockout efficiency for PD1 and B2M, and 96% for TRAC.