MICA, also known as MHC Class I Polypeptide-Related Sequence A, is a type I MHC molecule that is highly homologous to MICB. Both MICA and MICB lack the β2M subunit of classical MHC I, do not bind antigen peptides, lack the binding site for CD8, and do not participate in antigen presentation. MICA has nearly 100 allelic variants, and each variant exhibits different binding capacities with receptors, mediating varying degrees of effector cell responses. MICA is a stress-inducible protein and is rarely expressed in normal cells except in gastrointestinal epithelial cells, endothelial cells, and fibroblasts. However, its expression is significantly upregulated when cells are infected or undergo malignant transformation. Numerous studies have confirmed that MICA is highly expressed on the surface of various tumor cells, such as non-small cell lung cancer, colorectal cancer, and breast cancer, making it a tumor-associated antigen.
Exploring the Role of MICA in Tumor Immunity and Targeted Therapies
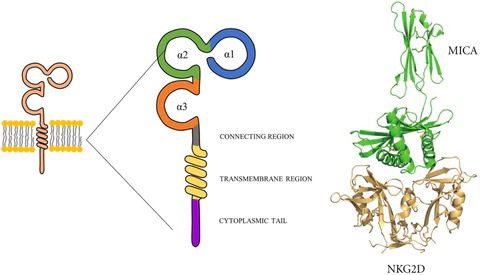
Figure 1. Schematic diagram of MICA structure and its binding to NKG2D [1, 2]
The α1 and α2 domains of MICA are responsible for binding to NKG2D. Proteases such as MMP and ADAM can cleave MICA into soluble sMICA. To evade immune cell killing, tumor cells can utilize their own proteases to cleave MICA, causing it to detach from the cell surface and lose its “identity card.” Shed MICA then binds to NKG2D on NK cells, inducing the internalization of NKG2D, which blocks the interaction between NK cells and MICA on the tumor cell surface, resulting in NK cell silencing and impairing their ability to recognize and kill tumor cells.
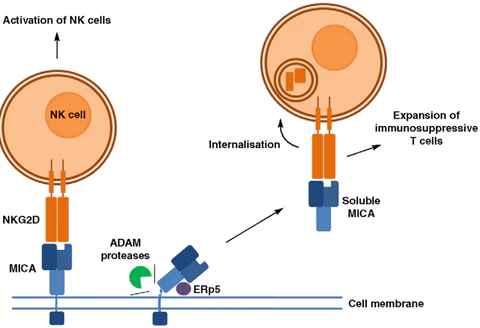
Figure 2. Mechanism of MICA shedding [3].
By designing antibodies against the α3 domain of MICA, it is possible not only to bind to MICA on the tumor cell membrane, preventing MICA shedding, but also to bind to free MICA, blocking the occupancy of sMICA, without affecting the binding of α1 and α2 domains to NKG2D. This ultimately activates NK cells, leading to the release of granulysin, perforin, chemokines, and cytokines, which can lyse tumor cells or induce tumor cell apoptosis. In addition to NK cells, NKG2D is also expressed on CD8+ T cells, so antibodies against MICA can also activate CD8+ T cells, further enhancing the anti-tumor immune response.
Clinical Drugs Targeting MICA
CLN-619
CLN-619, developed by Cullinan Oncology, is the first clinical-stage MICA antibody drug. CLN-619 exerts anti-tumor activity by preventing the shedding of MICA/B from tumor cells, antibody-dependent cell-mediated cytotoxicity (ADCC) effects, and enhancing the binding of MICA/B to NKG2D. Currently, it is in Phase I clinical trials in combination with Pembrolizumab for the treatment of solid tumors.
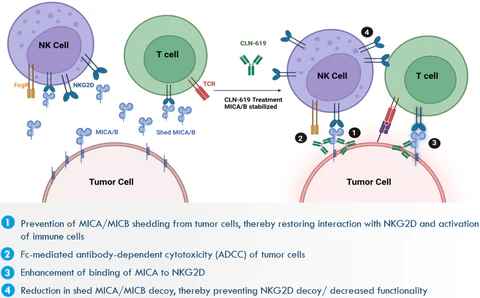
Figure 3. Mechanism of action of CLN-619 [4]
FT536
FT536 is a multi-functional, off-the-shelf CAR-NK cell therapy derived from induced pluripotent stem cells (iPSC). These cells express a CAR targeting the α3 domain of MICA/MICB, preventing MIC shedding. Additionally, FT536 expresses CD16 Fc receptor and IL-15 receptor fusion protein, enhancing antibody-mediated ADCC effects and the cytotoxicity of NK cells. Furthermore, FT536 has a CD38 knockout to sustain NK cell function in immunosuppressive microenvironments. Developed by Fate Therapeutics, FT536 is currently undergoing Phase I clinical trials.
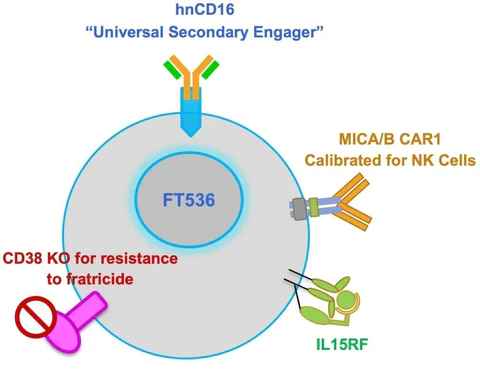
Figure 4. FT536 [5]
Additionally, research teams have directly designed tumor vaccines targeting MICA/MICB α3. These vaccines can stimulate the production of corresponding antibodies in the body, thereby altering the fate of MIC proteins being cleaved, increasing the levels of MICA/MICB proteins on the tumor surface, and enhancing the antigen-presenting ability of classical dendritic cells (cDC) as well as the cytotoxic function of T cells [6].
Drugs targeting MICA aim to activate or directly engage immune cells, harnessing their cytotoxic capabilities. This approach provides an additional layer of safety compared to traditional direct tumor-targeting therapies. It allows for synergistic attacks independent of tumor antigens and demonstrates efficacy against drug-resistant tumors, which is one of the reasons why immune cell therapy and immunotherapy combinations have garnered significant attention.
KACTUS has developed highly active MICA, MICA α3, and their receptor NKG2D protein products. These products cover multiple species and are designed with different tags. They are expressed in mammalian cells, ensuring a more authentic conformation and excellent biological activity. All products undergo rigorous quality testing and can be applied to immunological and screening studies of MICA drugs.
Product Validation
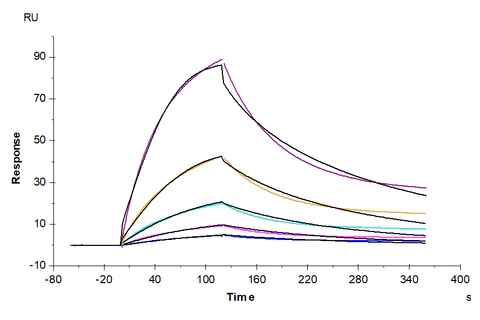
Human MICA, hFc Tag captured on CM5 Chip via Protein A can bind Human NKG2D, His Tag with an affinity constant of 0.36 uM as determined in SPR assay (Biacore T200).
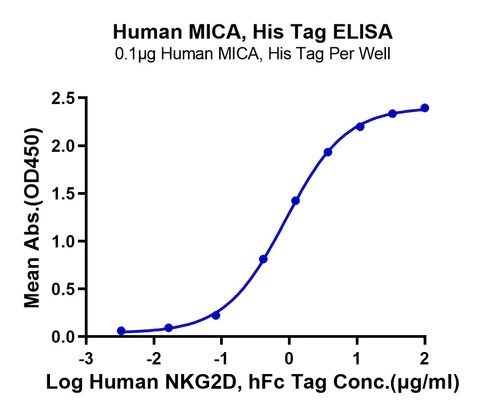
Immobilized Human MICA, His Tag at 1ug/ml (100ul/Well) on the plate. Dose response curve for Human NKG2D, hFc Tag with the EC50 of 0.88ug/ml determined by ELISA (QC Test).
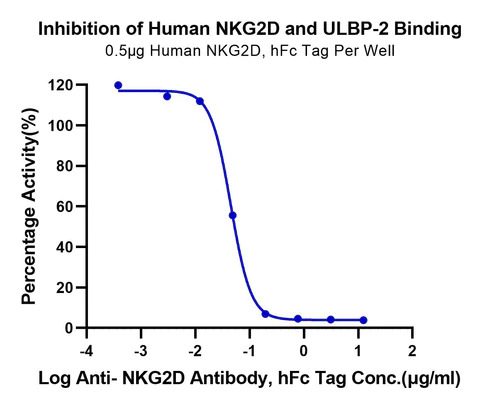
Serial dilutions of Anti-NKG2D Antibody, hFc Tag were added into Biotinylated Human ULBP-2, His Tag : Human NKG2D, hFc Tag binding reactioins. The half maximal inhibitiory concentration (IC50) is 45.2ng/ml.
MICA/NKG2D Product List
| Product | Tag | Catalog # |
| Human MICA | His-Avi | MIC-HM40A |
| Human MICA | hFc | MIC-HM20A |
| Biotinylated Human MICA | His-Avi | MIC-HM40AB |
| Cynomolgus MICA | His | MIC-CM10A |
| Human MICA alpha 3 | mFc | MIC-HM3AD |
| Human NKG2D | His-Avi | NKG-HM42D |
| Human NKG2D | hFc-Flag | NKG-HM22D |
| Biotinylated Human NKG2D | His-Avi | NKG-HM42DB |
| Mouse NKG2D | His | NKG-MM12D |
| Mouse NKG2D | hFc | NKG-MM22D |
| Cynomolgus NKG2D | His | NKG-CM12D |
References
[1] Machuldova A, Holubova M, Caputo VS, Cedikova M, Jindra P, Houdova L, Pitule P. Role of Polymorphisms of NKG2D Receptor and Its Ligands in Acute Myeloid Leukemia and Human Stem Cell Transplantation. Front Immunol. 2021 Mar 30;12:651751.
[2] Joyce MG, Sun PD. The structural basis of ligand recognition by natural killer cell receptors. J Biomed Biotechnol. 2011;2011:203628.
[3] Choy MK, Phipps ME. MICA polymorphism: biology and importance in immunity and disease. Trends Mol Med. 2010 Mar;16(3):97-106.
[4] https://www.cullinanoncology.com/
[5] https://fatetherapeutics.com/
