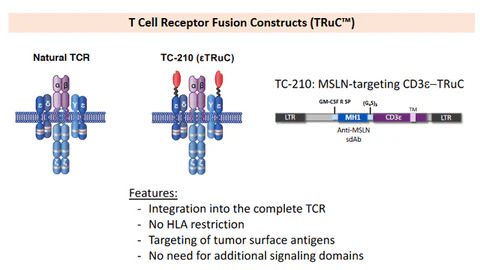
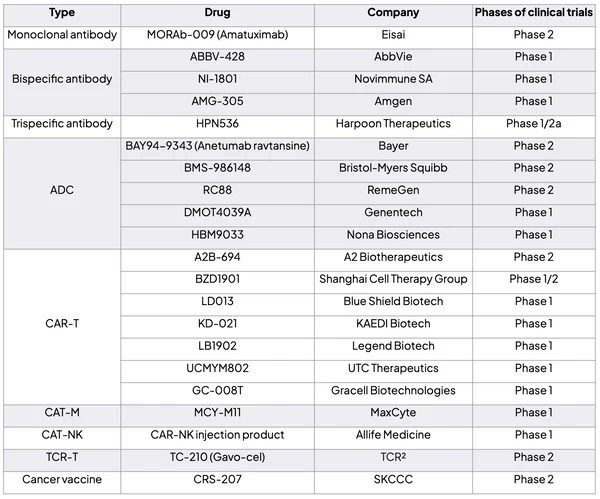
Since December 2023, there have been several positive updates about drugs targeting MSLN. On December 19th, RemeGen announced that the U.S. FDA has approved the Phase II clinical trial application of RC88 for treating platinum-resistant recurrent epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal cancer patients. This drug will be tested in multiple countries and regions including the United States, China, and the European Union. On December 15th, Nona Biosciences (a subsidiary of Harbour BioMed) signed a global clinical development and commercialization authorization agreement with Seagen (a subsidiary of Pfizer) for HBM9033. As part of the agreement, Nona Biosciences will receive a $53 million upfront payment and near-term payments, as well as up to $1.05 billion in milestone payments and royalty fees.
Expression and Function of MSLN
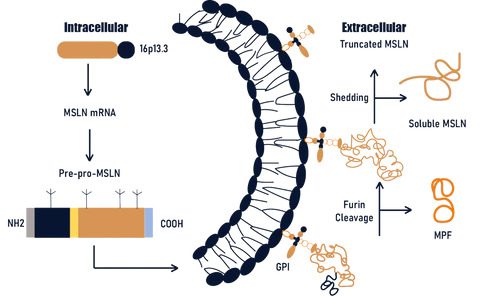
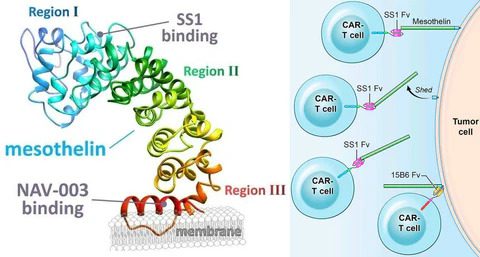
MSLN (Mesothelin) is a protein that is anchored to the membrane by GPI, without any transmembrane or intracellular regions. This protein can be cleaved by enzymes like Furin into two segments: a soluble form of approximately 31 kDa known as Megakaryocyte Potentiating Factor (MPF), and a mature form of around 40 kDa which remains tethered to the membrane. The mature form of MSLN can also be shed to produce soluble MSLN and truncated MSLN that remains attached to the