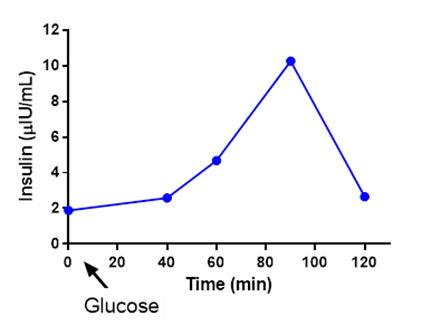
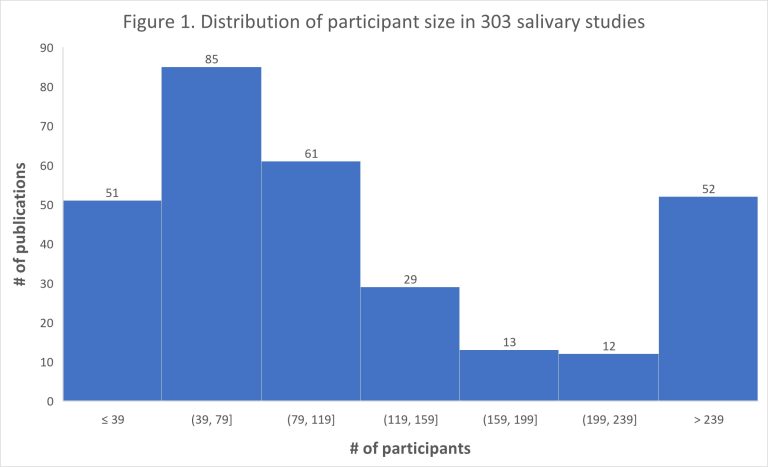
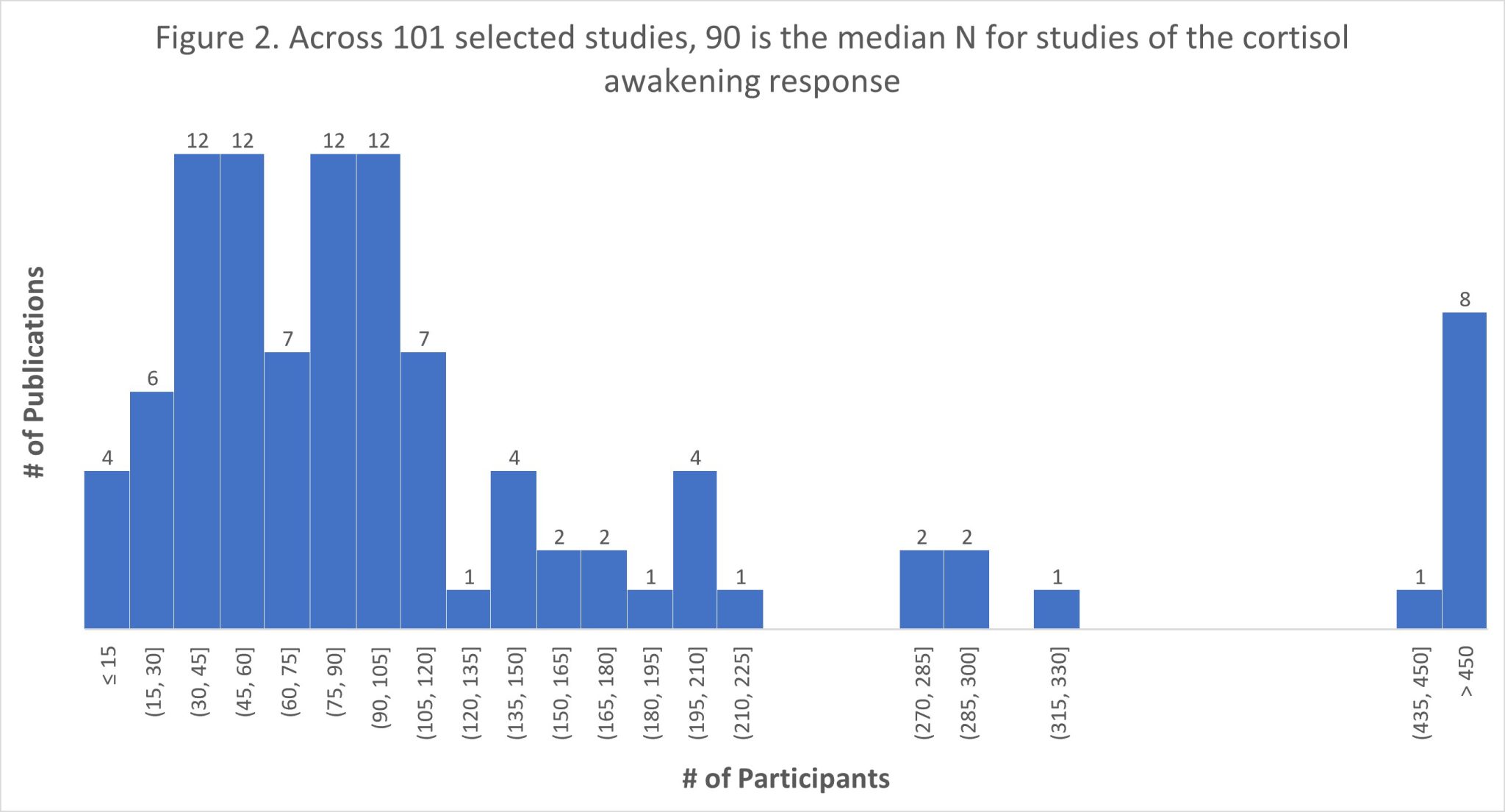
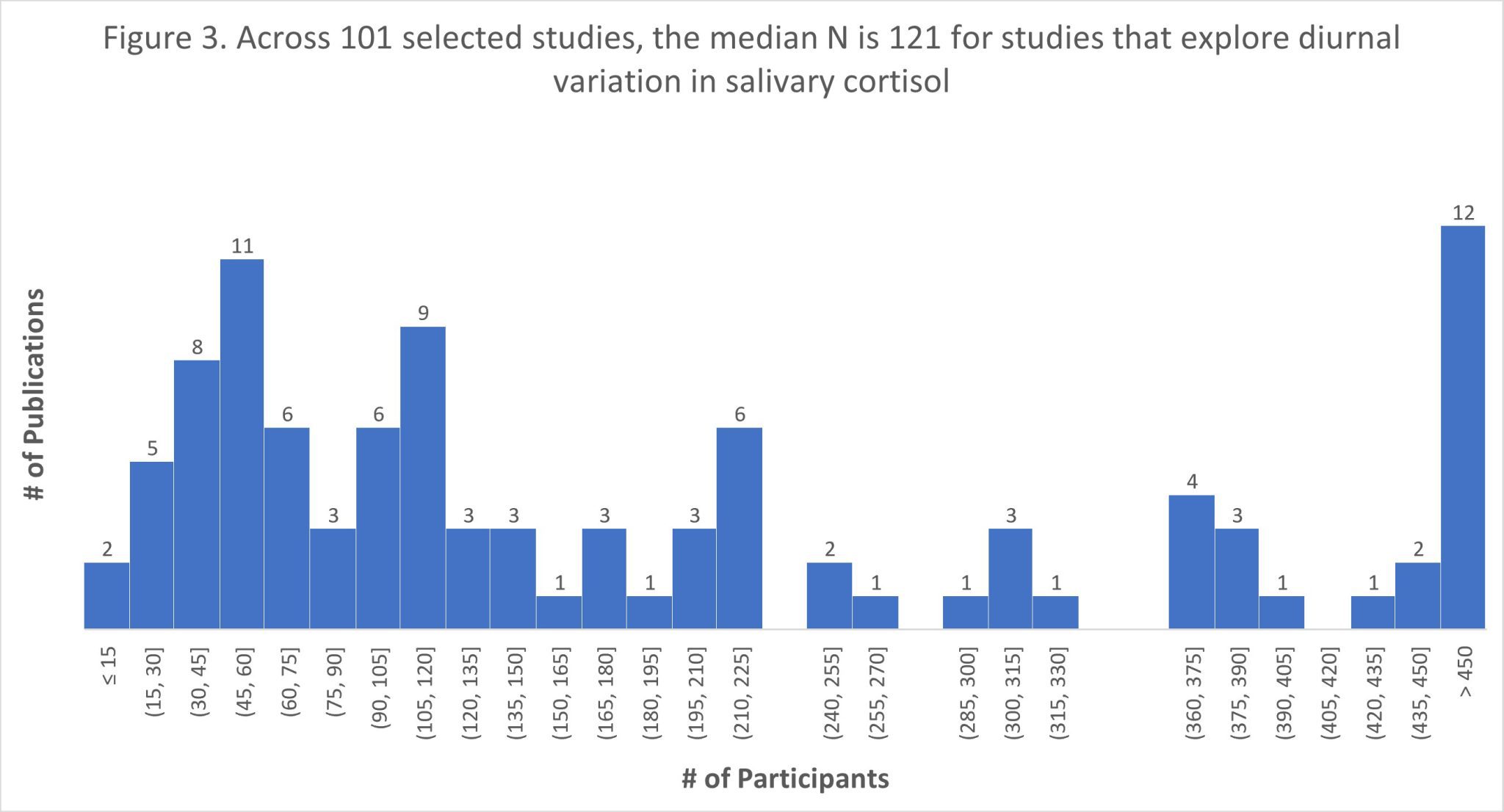
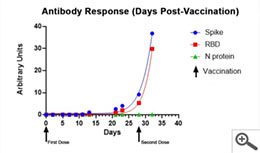
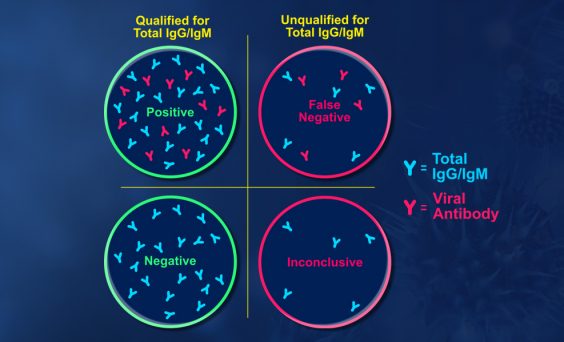
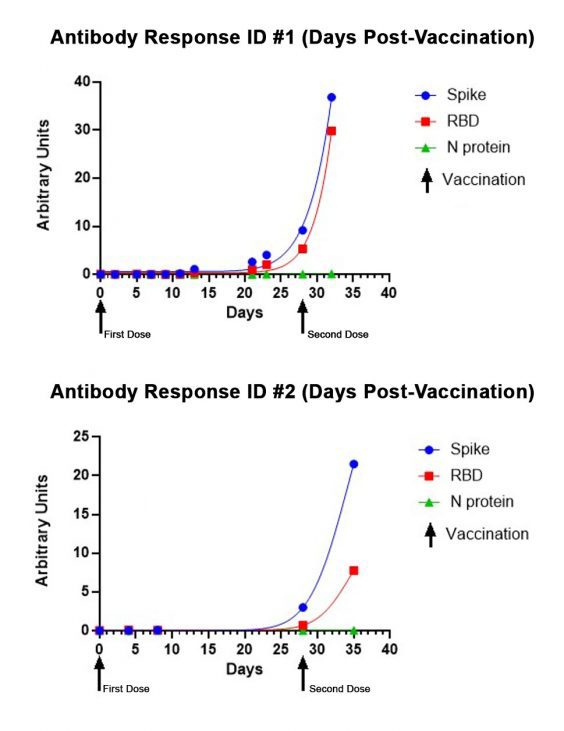
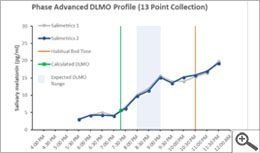
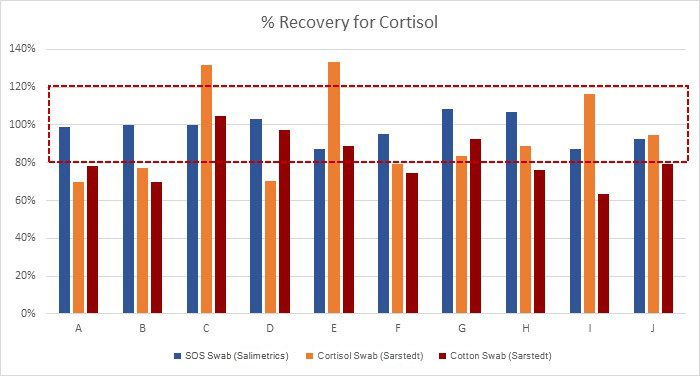
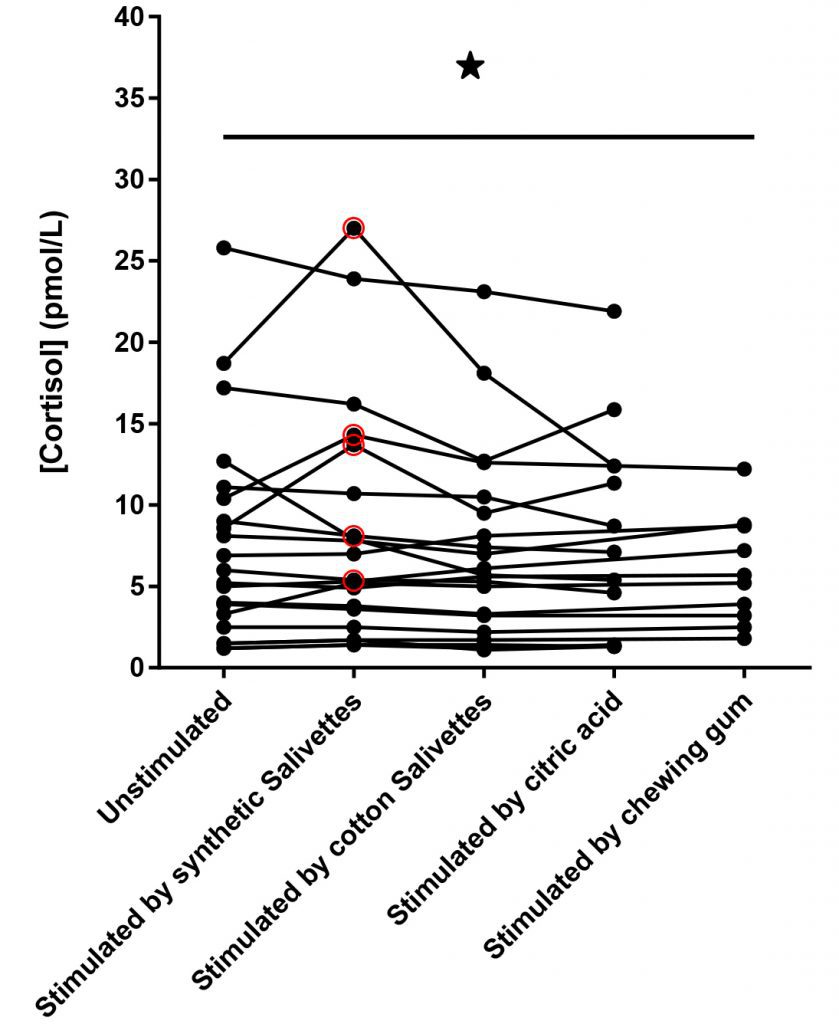
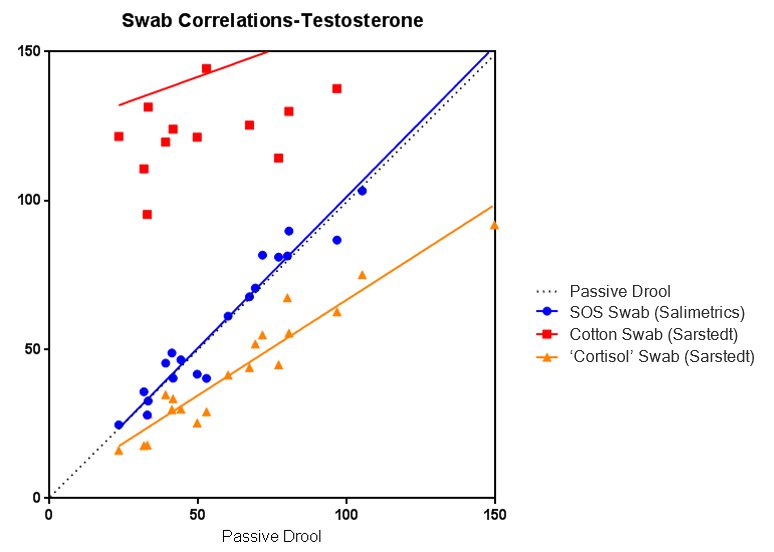
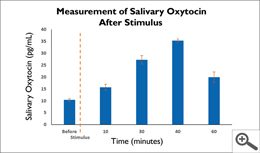
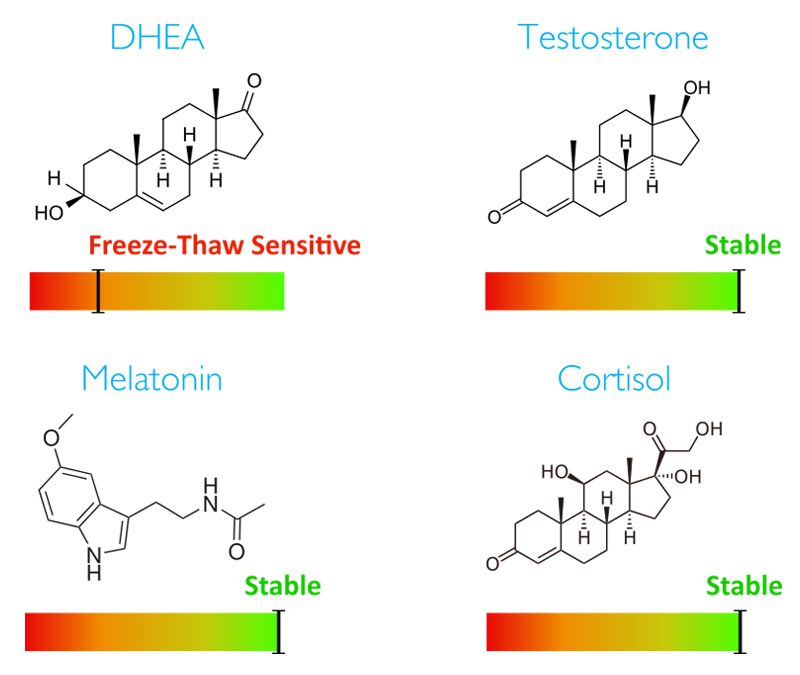
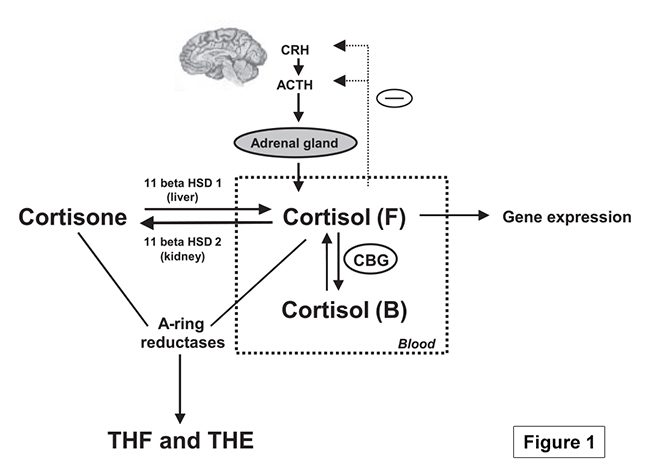
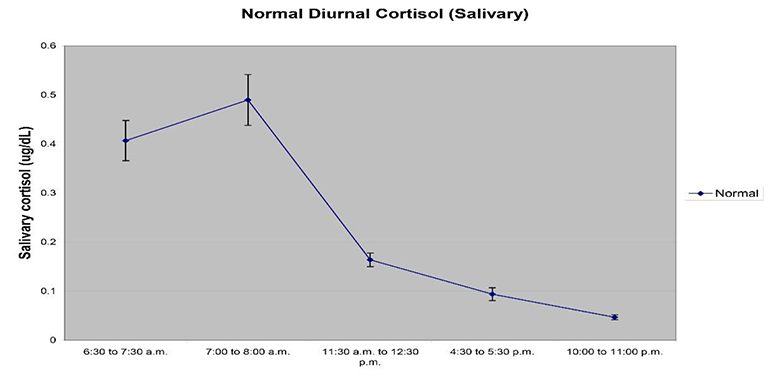
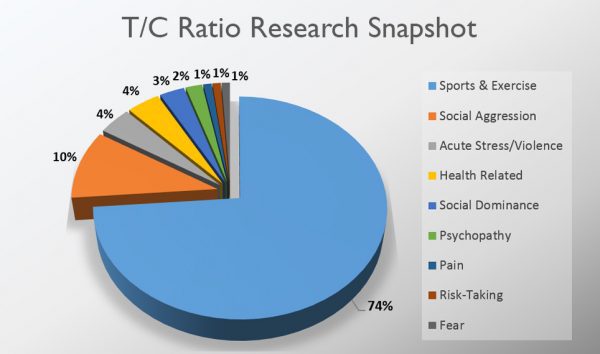
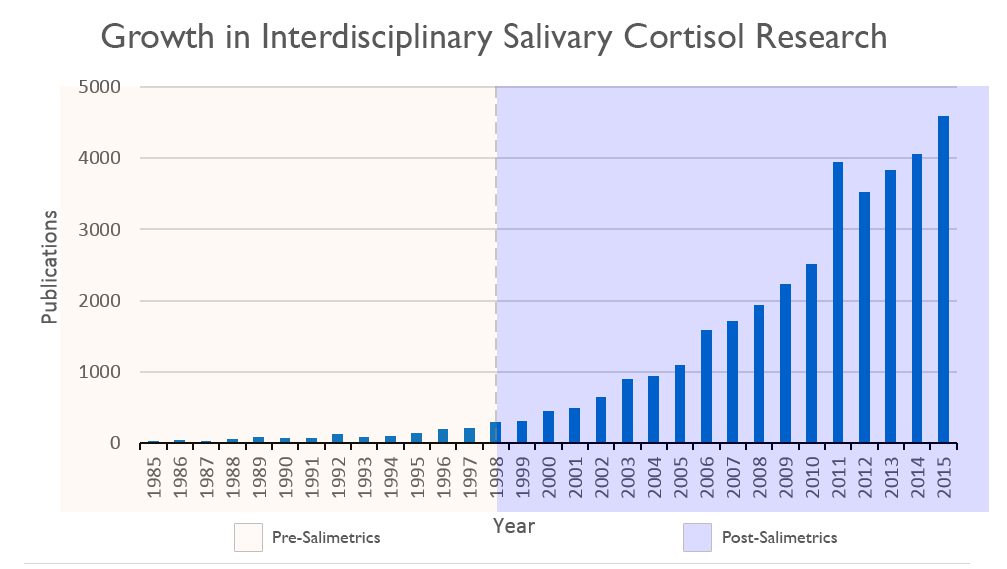
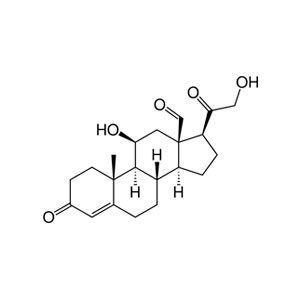
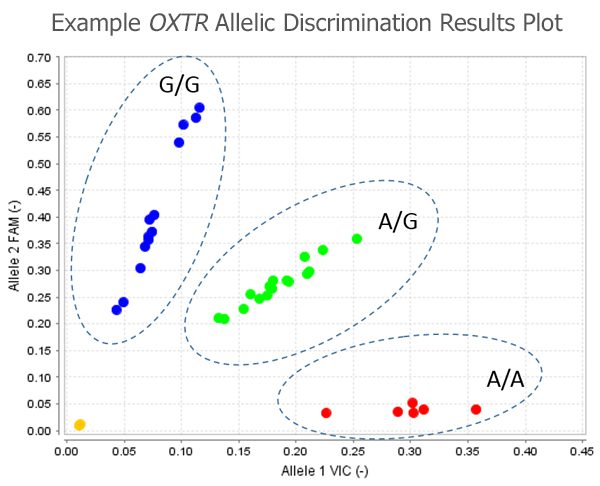
The scientific impact has been phenomenal and the collaborative environment, equal access, pay only when you need it strategy has allowed academics throughout the world to focus on what they do best while being backed by a team of salivary bioscience experts. This approach has saved institutions from building, maintaining, and decommissioning labs as resources and scientific foci change over time.
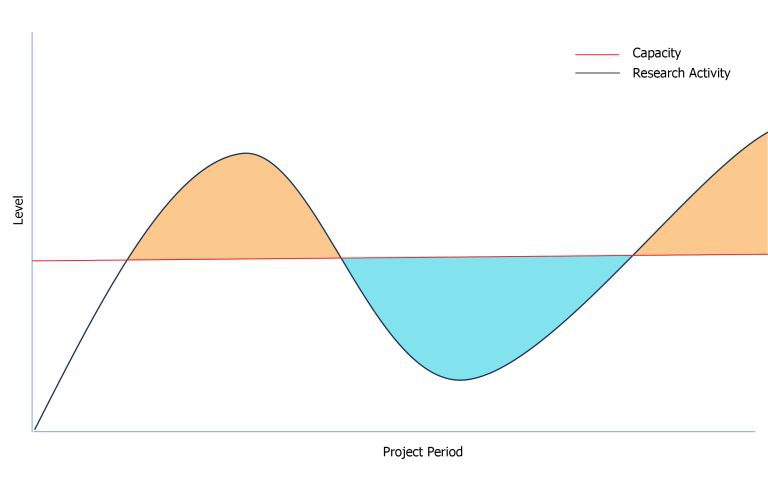
As a current or future principal investigator, it can be a major inefficiency (and distraction) to manage the “hidden costs” (time, effort, equipment maintenance, staffing, and local politics) associated with large, project-specific analytical laboratories. Organizing and maintaining operations of this scale may seem aspirational, but they can also hinder an investigator’s ability to focus on their research program deliverables (i.e., high impact novel scientific observations) and departmental expectations for service, teaching, and mentoring. It is also problematic when such a facility and staff are underutilized. That is, the costs to maintain a laboratory are perpetual, but the research activity is constantly transitioning between stages such as the project launch, data collection, laboratory analyses, interpretation, and scholarships.
In the beginning, the Salimetrics saliva testing laboratory was established to support and assist principal investigators. The purpose was to serve a community of scholars with common interests by establishing a shared, equal access facility that would be available to all, regardless of their specific academic affiliation or whether they were in-network with the laboratory’s lead scientist. The goal was to make the highest quality professionals, equipment, and knowledge available to all, as a consistent resource that would facilitate advancing the field by raising quality standards and minimizing inconsistencies between studies due to idiosyncratic methods.
Today, we still honor that mission. We also realize that it is hard to fully appreciate the Salimetrics laboratory experience without sending samples yourself, so we thought you might benefit from knowing just five of the many value-added features of the Salimetrics saliva testing laboratory.
FIVE ‘VALUE ADDS’ FOR PRINCIPAL INVESTIGATORS
1. DEPTH OF KNOWLEDGE
Salimetrics embodies these seven attributes of a high-quality salivary bioscience laboratory. In fact, Salimetrics pioneered the application of these criteria in saliva testing. The Salimetrics team is experienced and organized in handling even the most complex study designs. This experience has equipped us with a distinguished depth of knowledge in specific and specialized checks and balances that are designed to protect you and your research.
2. STANDARDIZING COLLABORATION FOR TEAM SCIENCE
It’s not us, it’s you. We are fortunate to be invited to play a specific, supportive role for research teams. The focus on collaboration and team science has helped launch hundreds of investigators’ careers and forms a strong network of interdisciplinary researchers worldwide. While it is very common that study designs are built off of previous work, there are regularly significant advances in the field. This knowledge is critical to designing an effective study that meets the current community guidelines and expectations. Imagine being backed by the latest collection, handling, transport, and testing protocols in less time than it takes you to do a single literature search! The Salimetrics team is always at the forefront of Salivary Bioscience so that you can feel confident in your collection, handling, transport, and testing methods.
3. A REAL ETA
Deadlines are important and the Salimetrics lab’s track record confirms that we can deliver results on time. Researchers won’t ever experience year-long turnaround times, broken freezers, improperly stored or misplaced samples, or even disappearing samples (We’ve heard a lot of crazy stories!). This can be especially vital to meet project deadlines, so you can reach the next stage of your research efficiently. You can rest easy knowing your samples are in safe hands here.
4. A STABLE, SHARED RESOURCE
For projects that require significant management or focus heavily on fieldwork, the Salimetrics laboratory is a well-trusted choice for researchers because it enables them to focus on the high-value inputs without the burden of monitoring, maintaining, resourcing, or transitioning lab staff and testing progress. At the Salimetrics lab, we employ only professional lab technicians, and each must adhere to strict GLP guidelines to ensure that each assay is properly run. Investigators don’t need to worry about changes in personnel, budget, experience, protocols, or capacity. This allows investigators to focus on details that make a study more credible, and not whether the lab staff ordered enough assay kits to properly run the samples, or if the kits they are using are expired (It happens more often than you would think!).
5. FLEXIBLE FOR PROJECTS LARGE AND SMALL, TESTING NOW OR LATER
With over 5,000 studies serviced, there is no study too big or too small. Whether there are 30 samples from one collection site or 10,000 samples from 24 collection sites, your project manager has seen it all before and can recommend logistical options to help you get your samples to the lab on time. In-person sample drop-off and rush testing are also available if you need results fast, and investigators can easily archive remaining samples for future testing on request. Salimetrics has also designed new programs to enable the exploration of novel salivary biomarkers through validated, early-stage methods which leverage know-how from Salimetrics to break new ground in salivary bioscience. If you want to know more about what’s new, connect with an expert!
THE BOTTOM LINE
In the end, Salimetrics saves investigators both time and money. In such a complex field, it is important to explore your project workflow from multiple perspectives to ensure that it fits with your research goals. Plus, if you ever need to connect with an expert, we’re here to help. While we recognize that there are times when a researcher may need to select a different lab, we highly recommend choosing a lab that is established, high quality, and has been properly vetted to comply with at least these seven attributes of a high-quality salivary bioscience laboratory.
*Note: Salimetrics provides this information for research use only (RUO). Information is not provided to promote off-label use of medical devices. Please consult the full-text article.