For more information about the reporter molecules available conjugated to JIR Affinity-Purified Secondary Antibodies and other products, including guidance on selecting the right conjugate for your experiment, select from the options below.
Secondary Antibody Conjugate Selection
Reporter Molecules Conjugated to Affinity-Purified Antibodies and Other Proteins
Fluorophores
JIR offers a wide range of fluorescent conjugates, covering the most commonly used excitation sources and filter sets from blue to infrared emissions. The following information describes some of the characteristics of fluorescent dye families and where they are best employed. Information about individual dyes can be found by selecting the dye from the table below.
Further information about selecting a fluorophore for your application is available on the fluorophore selection page.
Alexa Fluor® Fluorescent Dyes
Alexa Fluor® fluorescent dyes are widely recognized as superior fluorescent dyes, respected for their brightness and photostability. They are highly water soluble and remain fluorescent from pH 4 to pH 10.
Brilliant Violet™ Dyes
JIR offers two BD Brilliant Violet dyes, BV421 and BV480 (these dyes are named for their emission maxima, while many fluorophores are named for the excitation maxima). BV dyes are polymer chains and can be considered as a collection of optical segments, each with the ability to absorb light and emit fluorescence signal. This results in dyes that have a bright fluorescence signal for superior resolution and sensitivity.
Read more about Brilliant Violet™ conjugates.
Cyanine dyes (Cy™2, Cy™3 and Cy™5)
Among currently available fluorescent dyes, the cyanine dyes are better able to withstand the harsh dehydration and embedding conditions required for mounting sections in non-polar plastic mounting media such as DPX and Permount™. The cyanine dyes are brighter in the non-polar environment than in aqueous media, resulting in reduced acquisition time compared with DyLight™ and Alexa Fluor® dyes, even though those dyes are brighter in aqueous mounting media.
Read more about Cyanine conjugates.
Fluorescent Proteins – Phycoerythrin, PerCP and Allophycocyanin
Jackson ImmunoResearch offers 3 fluorescent proteins, Phycoerythrin (R-PE), Allophycocyanin (APC), and Peridinin-Chlorophyll-Protein (PerCP). R-PE and APC are light-harvesting phycobiliproteins found in red, blue-green and cryptomonad algae. Jackson ImmunoResearch offers R-PE in the form found in red macrophytic algae (seaweed). APC is isolated from the blue-green alga Spirulina. PerCP is a fluorescent peridinin-chlorophyll-protein complex isolated from dinoflagellates. R-PE, PerCP and APC can be excited by light over a wide range of the visible spectrum, are highly water soluble, have relatively low isoelectric points, and lack potentially sticky carbohydrates. It should be noted that the relatively high molecular weights of these fluorescent proteins may preclude their use in procedures requiring good penetration into cells and tissues. They are predominantly intended for surface labeling of cells for flow cytometry.
Read more about secondary antibodies for flow cytometry.
Fluorescent Conjugates Available from Jackson ImmunoResearch
The following seventeen fluorescent probes are currently available from Jackson ImmunoResearch. Click on each fluorophore in the table for more information.
| Fluorophore | Excitation Peak (nm) | Emission Peak (nm) |
| DyLight™ 405 | 400 | 421 |
| Brilliant Violet 421™ | 407 | 421 |
| Aminomethylcoumarin Acetate, AMCA | 350 | 450 |
| Brilliant Violet 480™ | 436 | 478 |
| Cyanine, Cy™2 | 492 | 510 |
| Alexa Fluor® 488 | 493 | 519 |
| Fluorescein, FITC/DTAF | 492 | 520 |
| Indocarbocyanine, Cy™3 | 550 | 570 |
| R-Phycoerythrin, R-PE | many, 488 | 580 |
| Rhodamine Red™-X, RRX | 570 | 590 |
| Alexa Fluor® 594 | 591 | 614 |
| Allophycocyanin, APC | many, 650 | 660 |
| Alexa Fluor® 647 | 651 | 667 |
| Indodicarbocyanine, Cy™5 | 650 | 670 |
| Peridinin-Chlorophyll-Protein, PerCP | many, 488 | 675 |
| Alexa Fluor® 680 | 684 | 702 |
| Alexa Fluor® 790 | 792 | 803 |
Spectra Viewer
Fluorescent probes or fluorophores (fluorescent dyes or proteins) are coupled to a secondary antibody or streptavidin to allow visualization of an analyte. Each fluorophore has its own spectral characteristics, with excitation and emission spectra particular to the molecule. Use the spectra viewer to build dye panels and compare the suitability of dyes for your application. Four dyes typically recommended for multiple labeling are pre-loaded, these can be changed as required.
Fluorescent probes or fluorophores (fluorescent dyes or proteins) are coupled to a secondary antibody or streptavidin to allow visualization of an analyte. Each fluorophore has its own spectral characteristics, with excitation and emission spectra particular to the molecule. Fluorescence facilitates the simultaneous detection of multiple analytes for a number of techniques including flow cytometry, fluorescence microscopy (epifluorescence, confocal, multiphoton and super-resolution techniques), Western blotting and ELISA.
The choice of fluorescent probe depends on a number of experimental variables, described below. There is also a Spectra Viewer Tool to assist in experimental design.
Further information about each of the fluorescent dyes offered is available on the Fluorophore Characteristics page.
Technique
Selection of a fluorophore depends on the intended application. Sample specifics influence the choice, as they may accommodate the use of particular fluorophores, and preparation may alter the way a fluorophore behaves. The following table lists some of the considerations which are pertinent to the choice of a fluorophore for a particular technique.
| Technique | Considerations |
| Flow cytometry | Surface staining accommodates the use of larger and brighter fluorescent conjugates such as the fluorescent proteins (R-PE, APC and PerCP) or Brilliant Violet™ dyes. Smaller fluorescent conjugates can be used for both surface and intracellular staining. |
| IHC microscopy |
|
| IHC multiple labeling |
|
| Super-resolution microscopy (STED and STORM) |
|
| Western and dot blotting |
|
Table 1: Considerations for conjugate selection.
Instrument capabilities
Consider excitation capabilities (lamp, lasers or LEDs determine excitation wavelengths) and the number of channels and filters available. Also consider the detection system, duration of data collection and post assay analysis.
Sensitivity required
The detection level of any fluorophore-antibody conjugate depends on brightness and photostability of the dye; antibody activity, specificity and cross-reactivity; and the optimal dye:antibody ratio (moles of dye per mole of antibody). These parameters have been researched for each of JIR’s dye conjugates to optimize the level of antibody detection and minimize background. The larger phycobiliproteins have high quantum yields (are very bright), but are limited to surface applications. Detection of poorly expressed analytes is enhanced by choosing a brighter fluorophore. For example, Alexa Fluor® 488 is brighter than FITC.
Experimental sample
Consider autofluorescence of the sample and possible expression of recombinant fluorescently tagged proteins. Choose fluorophores whose spectra do not overlap with endogenous fluorescence.
Degree of color separation required
For multiple labeling protocols, the dye panel choices will be constrained by instrumentation and sample specifics as described above. To achieve good color separation, choose fluorophores with minimal spectral overlap. The Spectra Viewer below can help build dye panels specific to any instrument.
Read more information on IHC multiple labeling
Spectra Viewer
Fluorescent probes or fluorophores (fluorescent dyes or proteins) are coupled to a secondary antibody or streptavidin to allow visualization of an analyte. Each fluorophore has its own spectral characteristics, with excitation and emission spectra particular to the molecule. Use the spectral viewer to build dye panels and compare the suitability of dyes for your application. Four dyes typically recommended for multiple labeling are pre-loaded, these can be changed as required.
Multiple labeling for simultaneous detection of several targets
Multiple labeling is the process of sequential immunolabeling to detect multiple antigens by either immunofluorescent or colorimetric IHC/ICC. The successful detection of more than one antigen requires rational experimental design, taking into account a large number of variables accumulated by each of the experimental steps. Here we detail how to set up the multiple labeling experiment and some considerations essential to executing a successful assay.
Designing the multiple labeling experiment
Selection of antibodies for simultaneous detection of more than one antigen depends on at least two important criteria:
- Availability of secondary antibodies that do not recognize:
- One another (are derived from the same host species),
- Other primary antibodies used in the assay system,
- Endogenous immunoglobulins present in the tissues or cells under investigation.
- Use of probes (enzyme-reaction products, fluorophores, or electron-dense particles) that are well resolved.
The affinity-purified antibodies marked “ML” (multiple labeling) have been specifically prepared to meet these criteria. They include mouse IgG subclass specific antibodies and antibodies cross-adsorbed (min X) against other species.
Multiple Labeling Example
| Tissue | Mouse | Mouse | Mouse |
| Antigen | Antigen A | Antigen B | Antigen C |
| Blocking Step | Step 1 5% Normal Donkey serum to block | Step 4 5% Normal Donkey serum to block (if needed) | Step 7 5% Normal Donkey serum to block (if needed) |
| 5% Normal Donkey serum to block | 5% Normal Donkey serum to block (if needed) | 5% Normal Donkey serum to block (if needed) | 5% Normal Donkey serum to block |
| Wash | Wash | Wash | |
| Primary Antibody Step | Step 2 Goat Anti-Antigen A | Step 5 Rabbit Anti-Antigen B | Step 8 Rat Anti-Antigen C |
| Wash | Wash | Wash | |
| Goat Anti-Antigen A | Step 3 Probe 1 Donkey Anti-Goat IgG (H+L) (min X Ck, GP, Sy Hms, Hrs, Hu, Ms, Rb, Rat Sr Prot) | Step 6 Probe 2 Donkey Anti-Rabbit IgG (H+L) (min X Bov, Ck, Gt, GP, Sy Hms, Hrs, Hu, Ms, Rat, Shp Sr Prot) | Step 9 Probe 3 Donkey Anti-Rat IgG (H+L) (min X Bov, Ck, Gt, GP, Sy Hms, Hrs, Hrs, Hu, Ms, Rb, Shp Sr Prot) |
Note: In this example, the secondary antibodies used do not recongnize each other since they are all made in donkey. They have been solid-phase adsorbed so that they do not recognize the other primary antibodies used (min X) in steps, 2, 5, and 8. Also, they do not react with endogenous mouse Ig, which may be present in mouse tissue.
Caution: Do not dilute any antibody with normal serum or mix antibodies together to save time, as this may result in immune complex formation and increased background.
Labeling primary antibodies from the same host species
Monovalent Fab fragments of affinity-purified secondary antibodies are offered to cover (block) the surface of immunoglobulins for double labeling primary antibodies from the same host species, or to block endogenous immunoglobulins in tissue sections or on cell surfaces. They can be used for these purposes because Fab fragments have only a single antigen binding site (i.e. they are monovalent).
Labeling primary antibodies from the same species as the sample
Sometimes it may be necessary to use a primary antibody from the same species as the sample, for example Mouse on Mouse. In these circumstances secondary antibodies may also detect endogenous immunoglobulin, causing unwanted background. This can be avoided with Fab fragment blocking.
Further Considerations
Blocking
For general blocking purposes, normal serum (5% v/v) from the same species as the secondary antibody host provides efficient background reduction for non-specific, conserved-sequence, and/or Fc-receptor binding.
Specific unwanted reactions with antibodies can be blocked with monovalent Fab fragments of secondary antibodies. This type of blocking is indicated for situations in which the specimen and primary antibodies are of the same species (e.g.mouse on mouse labeling), or when multiple primary antibodies are raised in the same host animal.
Visualization
Successful multiple labeling depends on the use of probes whose signals can be distinguished by available equipment.
Fluorescence microscopy is a common platform for multiple labeling, since filter sets have been designed to discriminate among the many fluorophores available. Narrow band-pass emission filters are critical for separating signals from multiple fluorophores, suppressing detection of fluorescence from overlapping spectra. When planning a multiple labeling protocol, formulate a dye panel from fluorophores with well separated emission spectra that are compatible with available instrumentation.
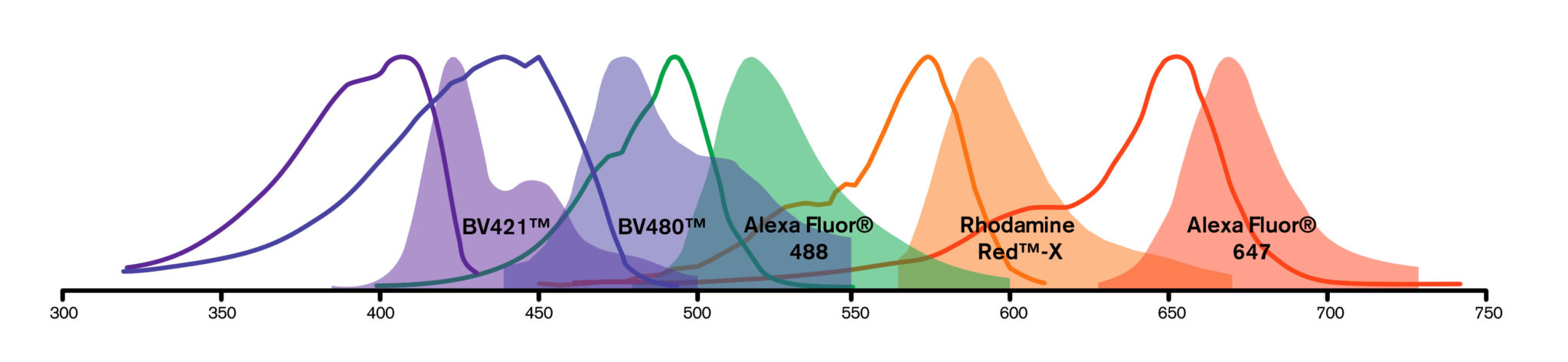
| Channel | Blue | Cyan | Green | Orange | Red |
| Option 1 | DAPI | BV480 | Alexa Fluor 488 | RR-X | Alexa Fluor 647 |
| Option 2 | BV421 | BV480 | Alexa Fluor 488 | RR-X | DRAQ5 |
| Option 3 | BV421 | BV480 | Alexa Fluor 488 | RR-X | Alexa Fluor 647 |
Multiple labeling can also be achieved with enzyme-linked antibodies. An antigenic site is labeled with a primary and secondary antibody, followed by color development with a chromogenic substrate such as DAB, TMB or AEC. Additional antigenic sites are labeled sequentially, with different chromogens used for each antigenic site.
For multiple labeling in electron microscopy, different sizes of colloidal gold particles complexed with secondary antibodies allow clear visualization of separate antigenic sites.
Controls
Prior to performing a multiple labeling protocol, optimize conditions for each primary/secondary antibody pair. Titrating both the primary and secondary antibody will identify conditions with low background and best positive signal. To demonstrate the specificity of each secondary antibody for its intended primary, attempt to label primaries with the “wrong” secondary antibodies (negative controls).
When using primary antibodies raised in the same host as the specimen species (e.g. mouse on mouse), controls can be used to determine the level of non-specific signal.
Guide to Multiple Labeling with Secondary Antibodies Poster
Enzymes & Other Probes
Horseradish Peroxidase and Alkaline Phosphatase
Secondary Antibody Reporter Enzyme Conjugates
Reporter enzyme-conjugated secondary antibodies can be used for both chromogenic and chemiluminescent detection methods when combined with an appropriate substrate. Enzyme conjugates can be applied to many immunotechniques to provide robust and sensitive detection.
JIR offers secondary antibodies and other reagents conjugated to horseradish peroxidase and alkaline phosphatase.
Horseradish Peroxidase (HRP)
This commonly used reporter enzyme is derived from the root of the horseradish plant (Armoracia rusticana). JIR HRP conjugates are prepared by a modified Nakane and Kawaoi procedure (1974).
HRP conjugates are suitable for all immunotechniques employing colorimetric and chemiluminescent detection methods, including Western blotting, immunohistochemistry and ELISA. Jackson ImmunoResearch provides a wide range of secondary antibodies, with comprehensive options for host and target species, as well as immunoglobulin specificities.
Horseradish peroxidase is available conjugated to:
- Whole IgG Secondary Antibodies
- F(ab’)2 Fragment Secondary Antibodies
- Anti-Fluorescein, Anti-Digoxin, and Anti-Biotin Antibodies
- Streptavidin
- ChromPure™ Purified Proteins from Normal Serums
We also offer Peroxidase-Anti-Peroxidase (PAP) as as an alternative labeling approach.
Note about endogenous peroxidase
Some tissues contain endogenous peroxidase-like enzymes which can react with peroxidase substrates, resulting in background staining. Pre-treatment of sample with hydrogen peroxidase will exhaust the endogenous enzyme activity, allowing clear detection of specific signal.
Cautions regarding reagent compatibility:
- Do not add sodium azide to solutions containing HRP. It will inactivate the enzyme.
See Blocking and Controls Guide for details on how to check enzyme and substrate reactivity. - If glycerol is added to extend shelf life of reconstituted product, confirm that glycerol is ACS grade or better. Lower grades of glycerol may seriously inhibit peroxidase enzyme activity.
Alkaline Phosphatase
Alkaline phosphatase (from calf intestine) conjugates are prepared by a method modified from Avremeas et al., (1978). The resulting conjugates contain heterogeneous, high molecular weight complexes. They are sensitive reagents suitable for solid-phase immunoassays such as ELISA and Western blotting. Although alkaline phosphatase conjugates are sometimes used for immunohistochemistry, penetration into tissues may be limited by their large sizes.
Alkaline phosphatase is available conjugated to:
- Whole IgG Secondary Antibodies
- F(ab’)2 Fragment Secondary Antibodies
- Anti-Fluorescein, Anti-Digoxin, Anti-Biotin, and Anti-HRP Antibodies
- Streptavidin
References
Paul K. Nakane, Akira Kawaoi (1974). Peroxidase-labeled Antibody A New Method Of Conjugation Journal of Histochemistry & Cytochemistry Vol 22, Issue 12, pp. 1084 – 1091. 10.1177/22.12.1084
Avremeas, S., Ternynck, T. And Guesdon, J.-l. (1978), Coupling Of Enzymes To Antibodies And Antigens. Scandinavian Journal Of Immunology, 8: 7–23. Doi:10.1111/J.1365-3083.1978.Tb03880.X
Biotin-SP™ (long-spacer) Conjugated Secondary Antibodies
Biotin is a small molecule which non-covalently binds to avidin and streptavidin with very high affinity. The affinity of the interaction makes biotin an excellent conjugate for detection when used in immunohistochemistry techniques. Biotin conjugates can be employed in signal amplification techniques.
Biotin-SP is our trade name for biotin with a 6-atom spacer positioned between biotin and the protein to which it is conjugated. When Biotin-SP-conjugated antibodies are used in enzyme immunoassays, there is an increase in sensitivity compared to biotin-conjugated antibodies without the spacer. This is especially notable when Biotin-SP-conjugated antibodies are used with alkaline phosphatase-conjugated streptavidin. Apparently, the long spacer extends the biotin moiety away from the antibody surface, making it more accessible to binding sites on streptavidin.

Biotin is available conjugated to:
- Whole IgG Secondary Antibodies
- F(ab’)2 Fragment Secondary Antibodies
- Fab Fragment Secondary Antibodies
- FabuLight™ – Fc Specific Fab Fragments for Labeling Primary Antibodies In Solution
- Anti-Fluorescein, Anti-Digoxin, and Anti-HRP Antibodies
- ChromPure™ Purified Proteins from Normal Serums
Biotinylated antibodies require additional reagents for visualization. We offer streptavidin and Mouse Anti-Biotin conjugated to fluorophores and enzymes.
IHC with Biotin-Conjugated Secondary Antibody and HRP-Streptavidin
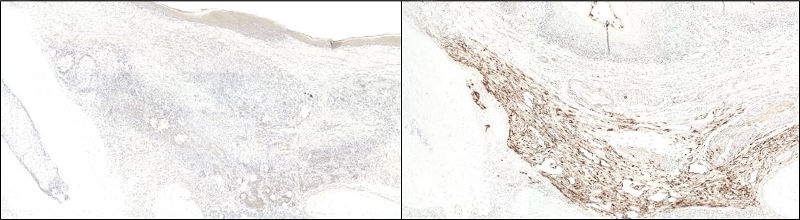
Anti-GFP immunohistochemical staining using Biotin-SP-conjugated Donkey Anti-Rabbit IgG (H+L) (711-065-152) secondary antibody followed by HRP-conjugated streptavidin (016-030-084):
(Left) At 4 weeks post-transplantation, no GFP signal could be detected in the in vivo specimens.
(Right) GFP-producing cells were visualized by brown staining in the positive control; signal was visualized using the chromogenic substrate 3,3′ diaminobenzidine (DAB). Nuclei were counter-stained with hematoxylin.
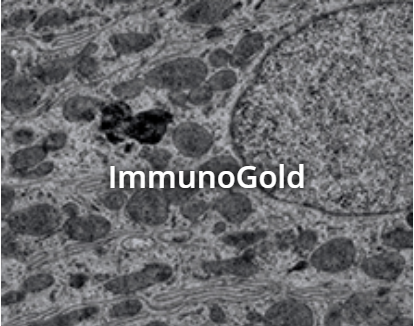
ImmunoGold Reagents
Introduction
Immunogold labeling or cytochemistry, is a technique used in Electron Microscope imaging and diagnostic devices such as lateral flow tests. A primary antibody for the protein of interest is detected by a secondary antibody conjugated to colloidal gold nanoparticles. Colloidal gold nanoparticles have a range of applications, when conjugated to antibodies these include high-resolution imaging and diagnostic uses. Different sizes of nanoparticle are available to cater for the technique employed.

Gold nanoparticles produce a phenomenon called surface plasma resonance (SPR) upon their interaction with light, leading to a colored output from the particle which is detectable by eye. The color ranges from bright red through to blue and infrared as the size of the particle increases. This property is exploited in their application to lateral flow immunoassays where a readout requires a colored output that is visible without special equipment.
The electron-dense nature of colloidal gold nanoparticles makes them ideal “marker” molecules for electron microscopy, and double labeling can be successfully performed by combining two sizes of particle, their sizes distinguishable under the microscope allowing differentiation of two target proteins without the need for fluorescence microscopy.
ImmunoGold colloidal gold reagents are available in a range of particle sizes allowing the appropriate size to be chosen for brightfield or electron microscopy, and lateral flow immunoassays.
40nm gold conjugates for Lateral flow immunoassays
Lateral flow Immunoassays (LFIAs) require a conjugate that generates a signal which can be read out by eye, but also requires physical properties that enable them to be used in single read formats. Each binding event should produce the strongest signal possible.
40nm colloidal gold conjugates are popular for LFIAs because they produce intense color. This makes readout easy, combined with the simplicity of their conjugation they offer a consistent and reliable reporter molecule.
Color intensity is due to the plasmon absorbance and light scattering characteristics of the gold’s electron shell (Huang & El-Sayed, 2010), and the particles’ ability to pack at high density on the test and control lines, due to their small size.
Antibodies are commonly conjugated with gold particles passively via electrostatic and hydrophobic interactions. The two entities are mixed in a low ionic strength buffer, followed by blocking with polyols or proteins like albumin or casein.
In addition to the antibodies listed here, we can also conjugate 40nm gold particles to other diagnostically relevant antibodies we produce, contact us to discuss your requirements.
As well as secondary antibodies, we provide Chrompure™ purified proteins conjugated to 40nm gold as control reagents.
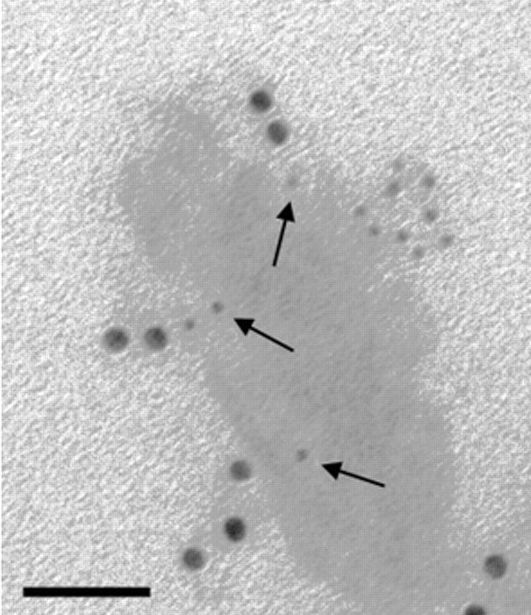
ImmunoGold reagents for electron microscopy
The EM Grade is distinguished from other commercial preparations by careful separation of monomeric particles from small aggregates using ultracentrifugation in density gradients. The resulting monomeric colloidal gold-protein complexes are recommended for multiple labeling applications, as different antigenic sites can be distinguished by particle size. The complexes are suspended in sterile-filtered buffer containing stabilizers and a preservative.
The 4 nm size may be used for electron microscopy in studies that require smaller particles since they are relatively uniform in size (coefficient of variation less than or equal to 15%), though small aggregates are not removed from this grade. The 4 nm particles are not suitable for multiple labeling with EM Grade reagents, since size uniformity is paramount and aggregated material may be mistaken for a larger particle.
Silver enhancement for light microscopy with ImmunoGold reagents
Silver enhancement allows the excellent penetration properties of ImmunoGold reagents to be used with light microscopy. The gold particles act as a nucleation site for the silver ions, which accumulate around the particle until enough contrast is generated to be visualized (Dixon et al., 2015). A detailed protocol for silver enhancement, using reagents that are easily prepared in the laboratory, is provided with all orders for LM Grade products. Alternatively, silver enhancement kits are commercially available.
Signal intensity is relatively independent of particle size when silver enhancement is used, so all particle sizes may be used for light microscopy or immunoblotting. For light microscopy, 4 nm particles (LM Grade) may penetrate tissues better than larger particles.
All LM Grade colloidal gold-protein complexes are freeze-dried in buffer with stabilizers and a preservative. After reconstitution, they may be frozen in aliquots for extended storage.
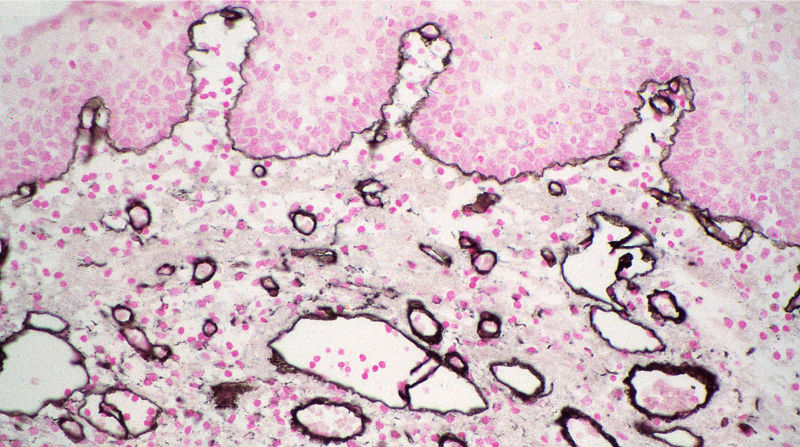
Figure 2. Immunolabeling for collagen type IV in normal human skin using ImmunoGold reagents 4 nm with silver enhancement. Prof. Jurgen Roth, Dept. Path. University of Zurich.
References
Dixon, A. R., Bathany, C., Tsuei, M., White, J., Barald, K. F., & Takayama, S. (2015). Recent developments in multiplexing techniques for immunohistochemistry. Expert Review of Molecular Diagnostics, 15 (9), 1171–1186. http://doi.org/10.1586/14737159.2015.1069182.
Timothy D. Blalock, et al; Functions of MUC16 in Corneal Epithelial Cells. Invest. Ophthalmol. Vis. Sci. 2007;48(10):4509-4518