Strep-tag® technology for cell isolation
The Strep-tag® technology provides methods for isolating cells via their surface markers (Traceless Affinity Cell Selection – Fab-TACS®/Nano-TACS®) or via their antigen specificity (MHC I Streptamer®).
The Strep-tag® technology provides methods for isolating cells via their surface markers (Traceless Affinity Cell Selection – Fab-TACS®/Nano-TACS®) or via their antigen specificity (MHC I Streptamer®).
The Strep-tag® technology allows label-free positive cell isolation targeting surface markers via three different methods: Affinity chromatographic, magnetic and fluorescent cell separation. All three approaches are called Fab-TACS®/Nano-TACS®, describing the underlying principle of label-free positive cell selection. “Fab” and “Nano” deviate from the central components used in all approaches: Antibody-derived Fab fragments or nanobodies that are fused to a Twin-Strep-tag® and specifically bind to a target cell of interest.
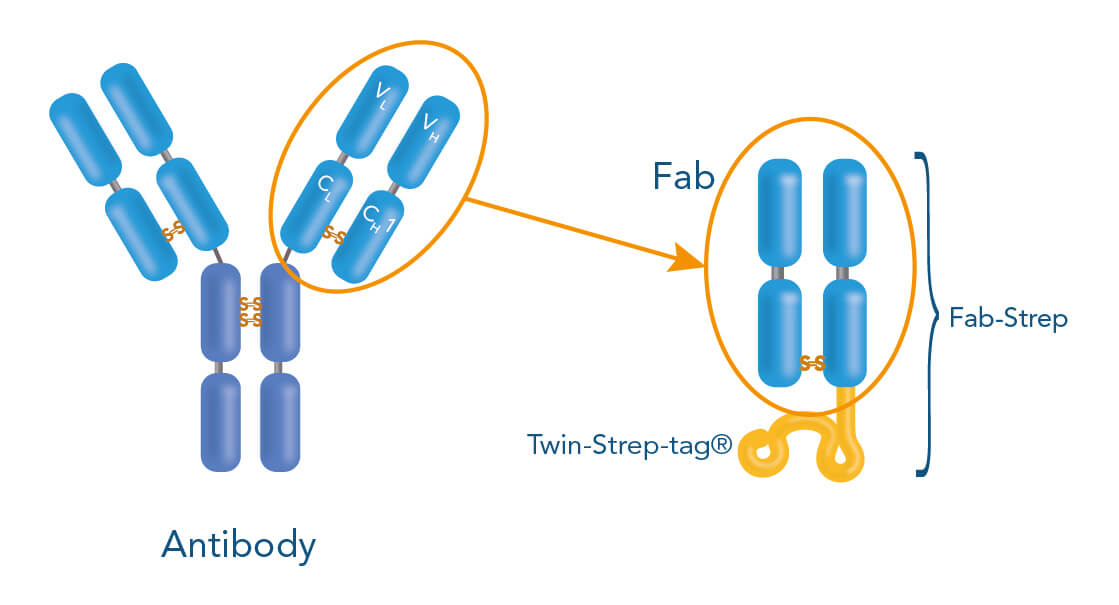
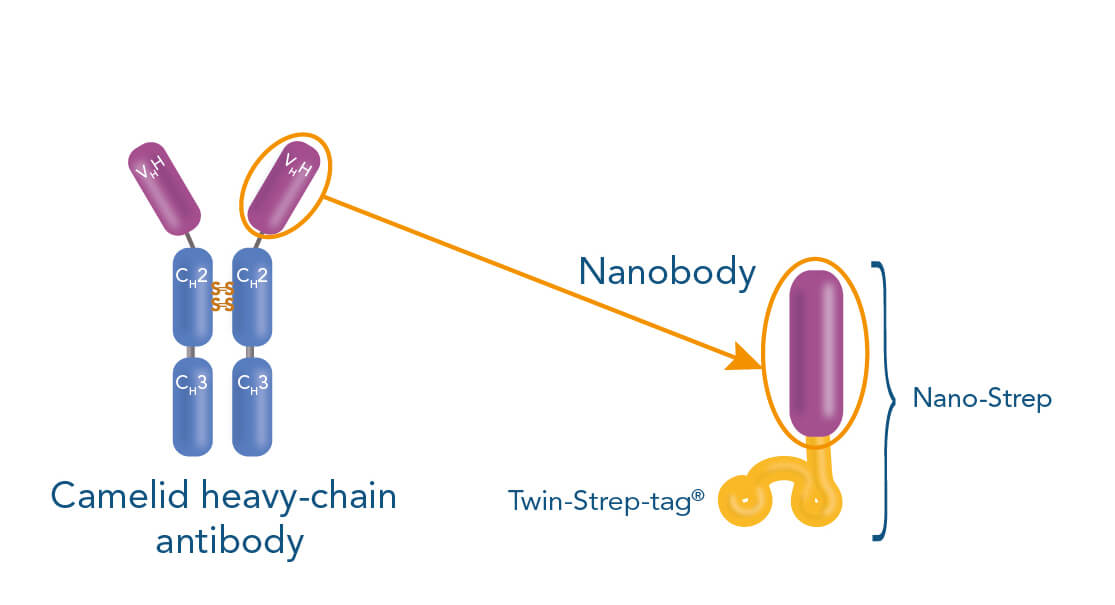
The Twin-Strep-tag® facilitates the binding of either Fab- or Nano-Streps to Strep-Tactin®, an engineered streptavidin. Different Strep-Tactin® conjugates are the basis for the three cell isolation approaches. For affinity chromatography, Fab- /Nano- Streps of choice are loaded into Strep-Tactin® TACS Agarose-containing columns. For magnetic cell isolation, Fab-/Nano-Streps are bound to Strep-Tactin® conjugated to magentic microbeads, whereas combining them with fluorophore-conjugated Strep-Tactin® allows fluorescent cell sorting.
Find application examples of Fab-TACS® affinity chromatographic isolation and Fab-TACS® magnetic isolation here.
Find Strep-Tactin® TACS Agarose columns, magnetic microbeads and fluorophore-conjugated Strep-Tactin® in our web shop.
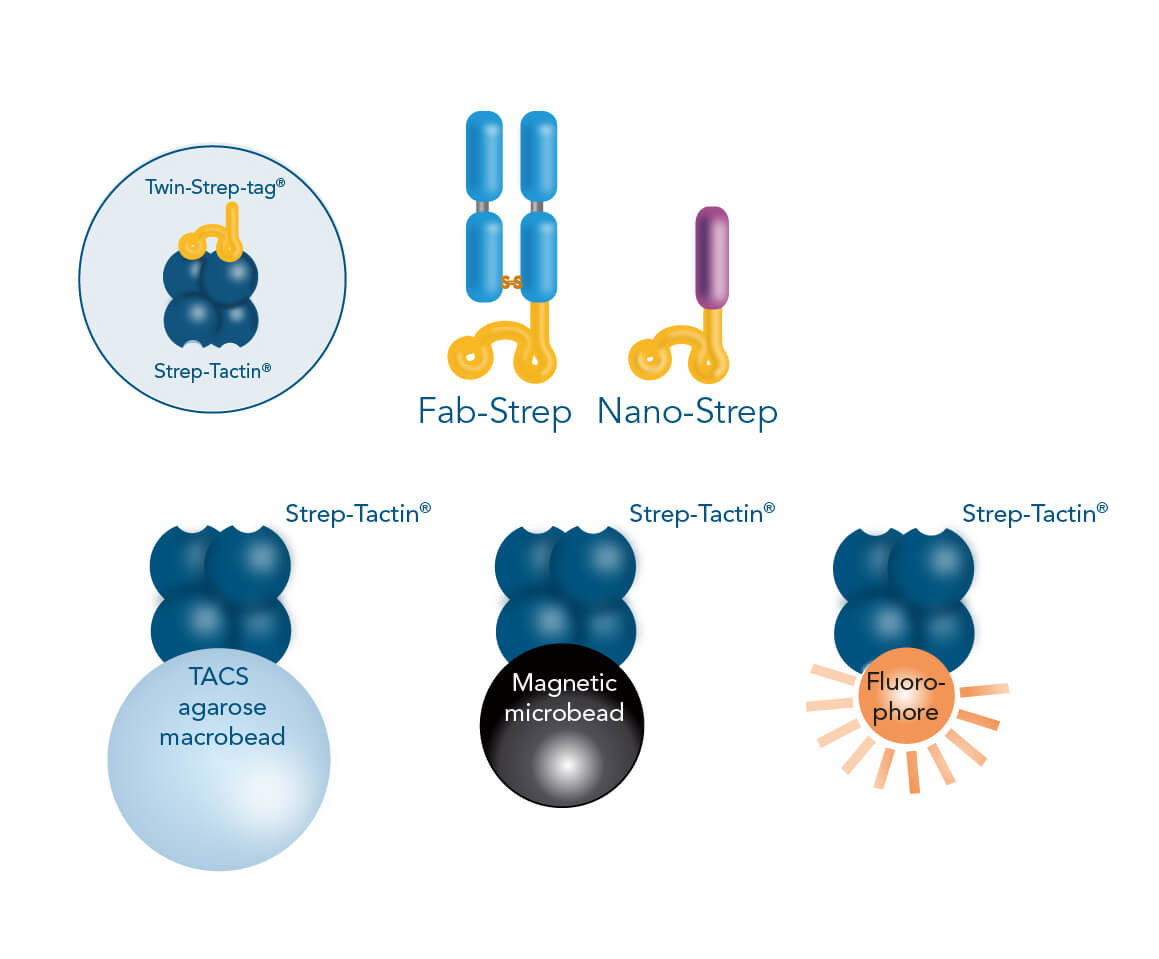
In the Fab-TACS®/Nano-TACS® approach, low affinity Fab-Streps or nanobodies against specific surface markers are multimerized on different Strep-Tactin® backbones to facilitate three isolation methods: affinity chromatographic isolation using TACS Agarose, magnetic isolation using Strep-Tactin® conjugated to magnetic microbeads and fluorescent cell sorting using Strep-Tactin® conjugated to fluorophores.
The key feature of the Fab-TACS®/Nano-TACS® principle is the removability of all labeling reagents. Two factors account for this feature: Low affinity Fab-/Nano-Streps and the reversible binding of the Twin-Strep-tag® to Strep-Tactin®.
The Fab fragments and nanobodies bind specifically, but only weakly, to the target. To achieve sufficient binding to the cells, Fab-/Nano-Streps are multimerized on Strep-Tactin® molecules to increase the avidity. Biotin addition reverses this binding. Due to its higher affinity, biotin replaces the Twin-Strep-Tag® on Strep-Tactin® molecules and thereby causes the release of Fab-/Nano-Streps. Due to their low affinity, Fab-/Nano-Streps spontaneously dissociate from the cell surface after being monomerized, resulting in a completely label-free cell population. Cells are only minimally affected during the isolation procedures and suitable for various downstream applications.
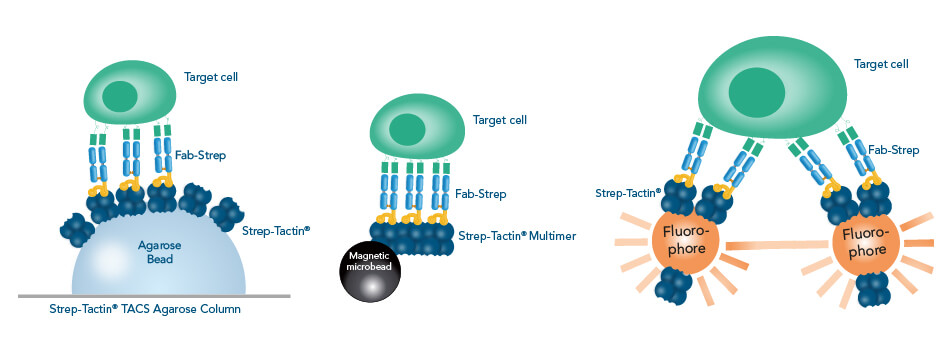
Due to their low affinity, a multimerization of Fab-Streps is required to increase the avidity sufficiently to immobilize target cells. This is achieved by using agarose beads coated with several Strep-Tactin® molecules that are filled into a column, by using Strep-Tactin® multimers conjugated to magnetic microbeads or by cross-linking Strep-Tactin® molecules with large fluorophores..
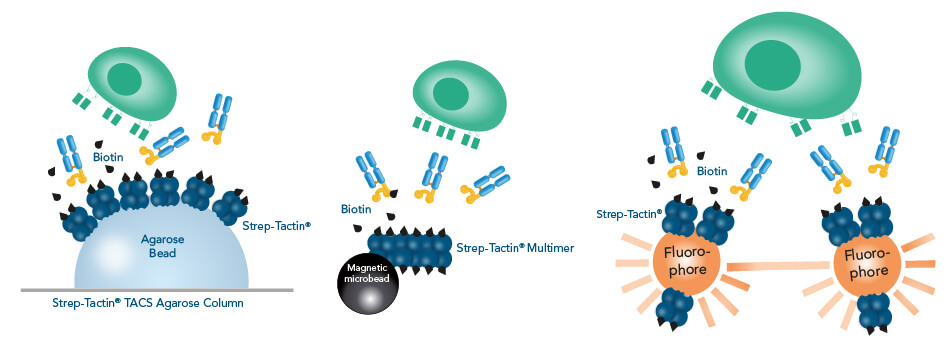
All Fab-TACS®/Nano-TACS® cell isolation methods use reversible reagents to generate label-free cells. Adding biotin releases the Fab-Streps from Strep-Tactin® and causes their spontaneous dissociation from the cells due to their low affinity as monomers.
In the Fab-TACS® approach that uses affinity chromatography as underlying isolation method, Fab-Streps of choice are loaded into columns containing an agarose coated with Strep-Tactin® that is suitable for traceless affinity cell selection (TACS). After the sample is applied, unwanted material is washed away. Finally, cells are eluted/released by adding biotin. Due to the low affinity of the Fab-Streps, they spontaneously dissociate from their target, yielding completely label-free cells.
In addition to providing a surface marker-specific cell selection approach (Fab-TACS®/Nano-TACS®), the Strep-tag® technology also facilitates label-free cell isolation according to antigen specificity. Three different methods are also possible here: Affinity chromatographic (for target cell numbers > 1 x 107), magnetic and fluorescent cell separation. They are summarized as MHC I Streptamer® approaches, describing the underlying principle of reversibly multimerizing MHC I complexes. Central in all three methods are peptide-loaded MHC I molecules that are fused to a Twin-Strep-tag®. They are multimerized on different Strep-Tactin® backbones, generating MHC I Streptamers. They specifically capture CD8+ T cells according to their antigen specificity.
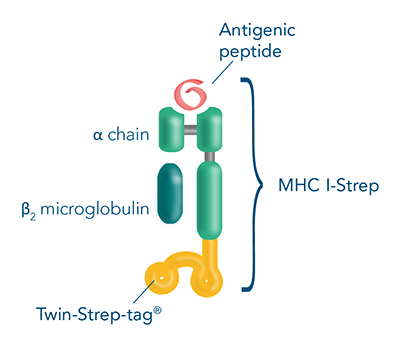
MHC I-Streps are MHC I-peptide complexes fused to a Twin-Strep-tag®. Combining MHC I-Streps with Strep-Tactin® molecules generates MHC I Streptamers that stably bind to antigen-specific T cells.
Similar to Fab-/Nano-Streps, the Twin-Strep-tag® facilitates the binding of MHC I-Streps to Strep-Tactin®, an engineered streptavidin. Different backbone-molecules conjugated to Strep-Tactin® distinguish the three cell isolation methods. In the first method, affinity chromatographic cell isolation, MHC I-Streps are loaded into columns that contain agarose macrobeads coated with Strep-Tactin®. Strep-Tactin® conjugated to magnetic microbeads is used for the second method, permitting magnetic cell isolation. Combining MHC-Streps with fluorophore-conjugated Strep-Tactin® provides the third possible method: fluorescent cell sorting.
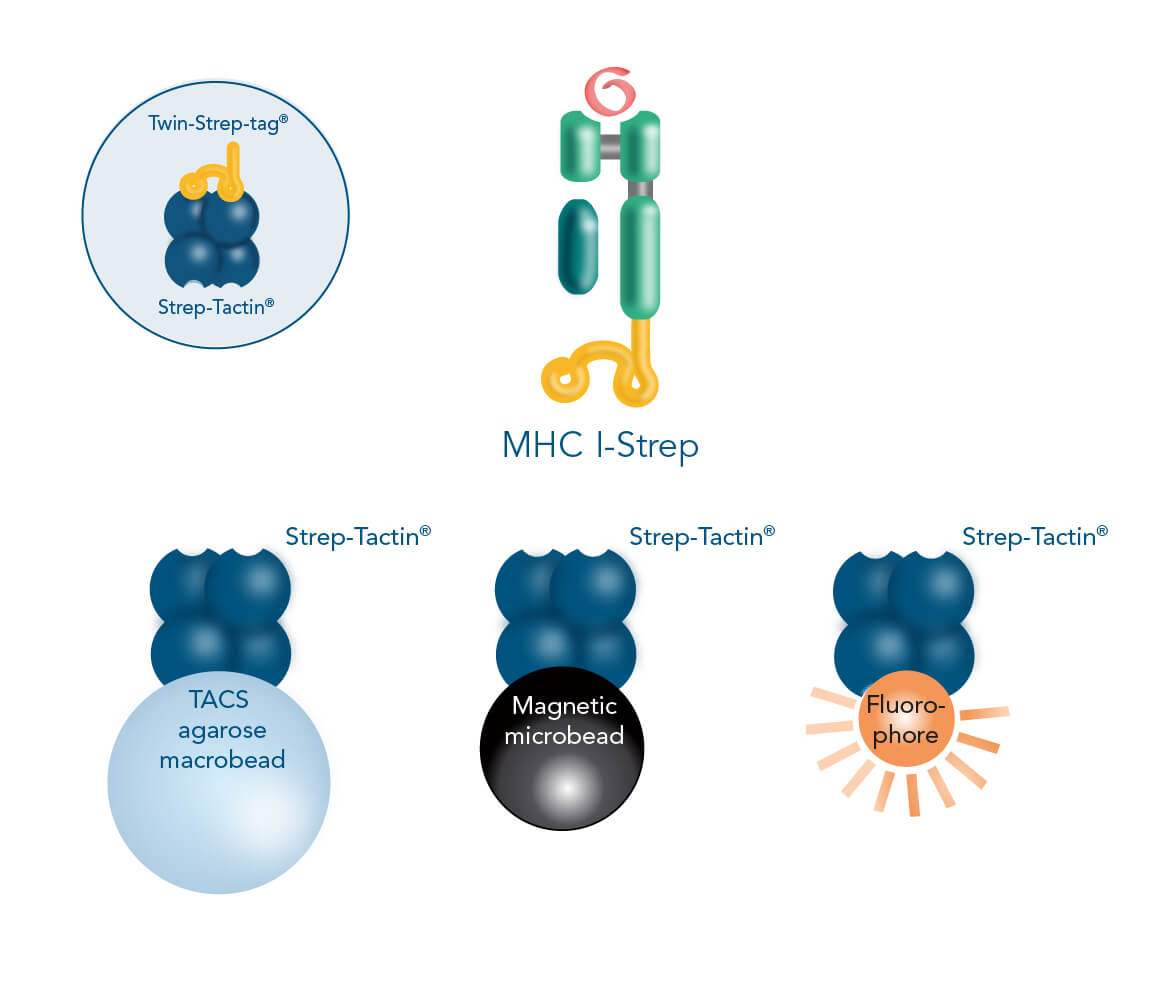
In the MHC I Streptamer® approach, MHC I-Streps targeting antigen specific T cells are multimerized on different Strep-Tactin® backbones to facilitate three isolation methods: affinity chromatographic isolation using TACS Agarose, magnetic isolation using Strep-Tactin® conjugated to magnetic microbeads and fluorescent cell sorting using Strep-Tactin® conjugated to fluorophores.
The key feature of MHC I Streptamer® isolation is the same as for Fab-/Nano-TACS®: the possibility to remove all labeling reagents after successful isolation. Low affinity of monomeric MHC I-peptide complexes to the T cell receptor and the reversible binding of the Twin-Strep-tag® to Strep-Tactin® are two factors that contribute to this feature. Biotin addition initiates the dissociation of all reagents from the cells. Due to its higher affinity, biotin replaces the Twin-Strep-Tag® on Strep-Tactin® molecules and thus causes the release of MHC I-Streps.
This reverses the multimerization of MHC I-peptide complexes and leads to their spontaneous dissociation from the cell surface due to their low affinity as monomers. The resulting cell population is completely label-free. This keeps the effects of the isolation procedure on the cells to a minimum, making them suitable for various downstream applications. The benefit of label-free cells has been shown in multiple scientific publications.
The following application note demonstrates how MHC I Streptamer® and Fab-TACS® approaches can be combined for tandem cell isolation.
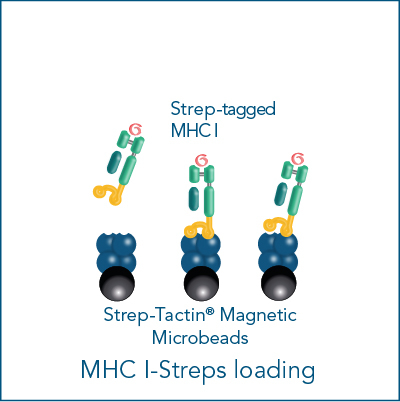
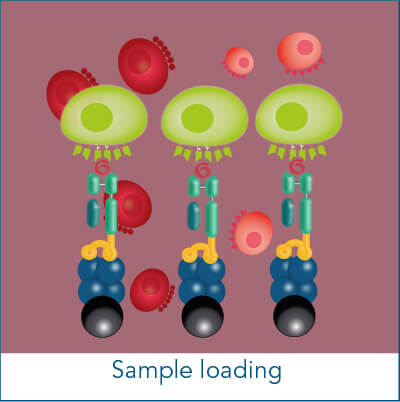
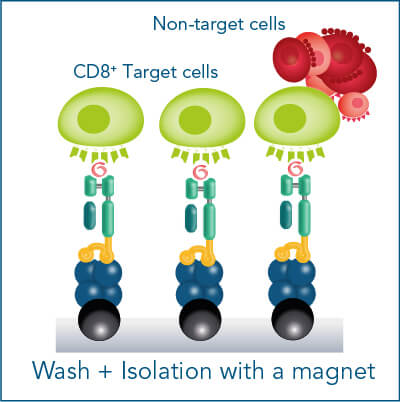


The MHC I Streptamer® magnetic cell isolation workflow includes the following steps: MHC I-Streps have to be loaded on Strep-Tactin® Magnetic Microbeads. The resulting complexes are used to label cells for subsequent positive selection on a magnet. After washing away all unwanted cells, biotin addition releases MHC I-Streps from Strep-Tactin® Magnetic Microbeads and causes the spontaneous dissociation of MHC I-Streps from the cell surface (elution).
The Strep-tag® technology offers two possibilities to isolate cells: Via their surface markers or via their antigen specificity. Fab-Streps or Nano-Streps that target specific surface markers are the central components in Traceless Affinity Cell Selection – Fab-TACS®/Nano-TACS®. In turn, MHC I-Streps are employed for the isolation of antigen-specific T cells in our MHC I Streptamer® approach.
For both approaches, three different methods are possible:
The method of choice depends on several factors such as target cell type, sample source/complexity, cell numbers and number of samples that need to be processed at the same time.
All methods are suitable for isolating cells from single cell suspension such as peripheral blood mononuclear cells (PBMCs) or murine splenocytes. Affinity chromatography based cell separation is best suited if a high amount of target cells (approximately > 1 x 107) or starting cell number in general is expected, whereas magnetic cell separation and fluorescent cell separation are advantageous for lower cell numbers.
Due to its applicability to a high amount of cell numbers, the affinity chromatography method also allows isolation of cells directly from whole blood or buffy coat. Find out more in our application example in which CD3+ T cells were isolated from buffy coats or murine splenocytes.
All isolation methods result in completely label-free cells that are suitable for downstream applications such as cell culture or in vivo experiments.
For further convenience, the affinity chromatographic method for surface marker-specific cell isolation is also available as automated system that uses the FABian® device. For more information about the automated cell isolation device, please contact: technical@stratech.co.uk
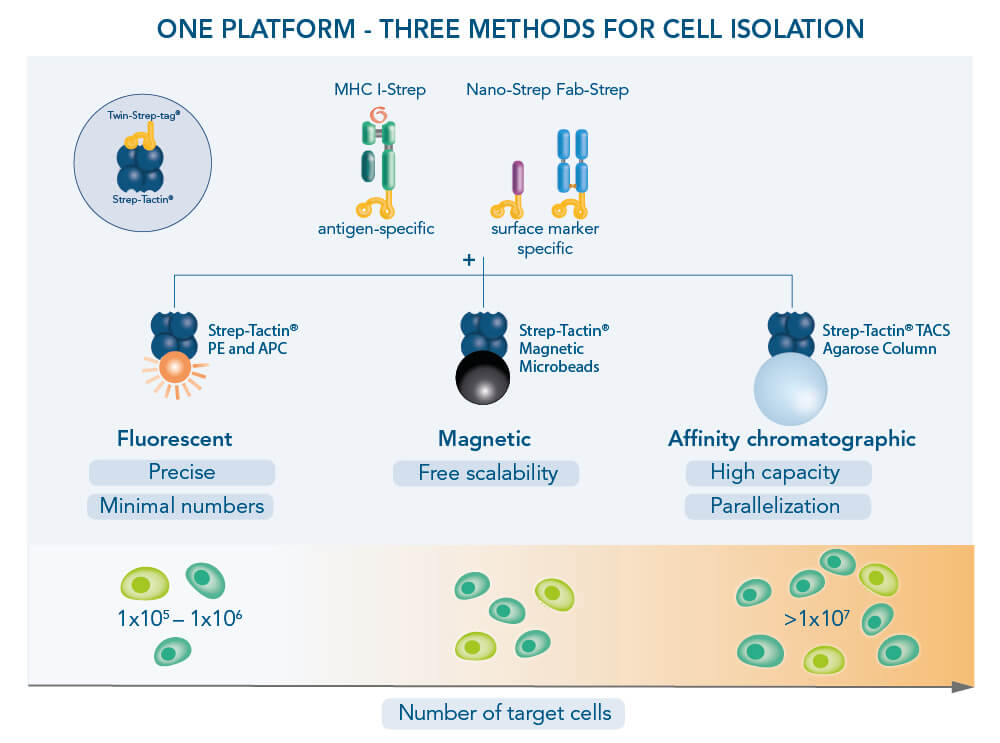
The Strep-tag® technology for cells offers flexible cell isolation approaches. The technology provides three principal cell isolation methods: affinity chromatographic, magnetic or fluorescent cell separation. The optimal method depends among others on target cell type and sample size.
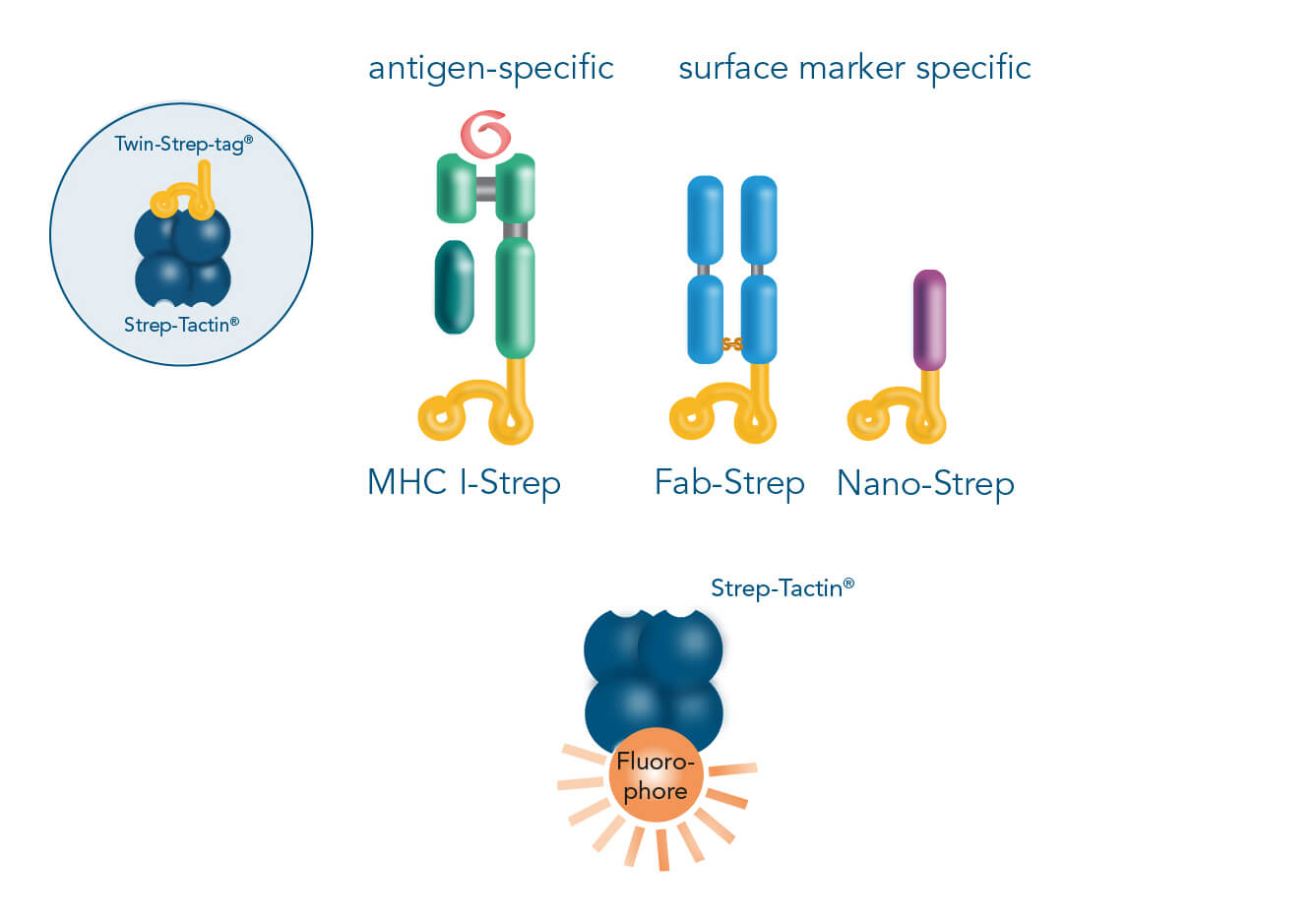
For cell staining, MHC I-Streps (antigen-specific T cells) or Fab/Nano-Streps (surface marker-specific) can be combined with fluorescent Strep-Tactin® via their Twin-Strep-tag®.
In addition to cell isolation, the Strep-tag® technology is also applicable to staining cells via their surface markers or according to their antigen specificity. The underlying principles for cell staining are the same as for cell isolation: Traceless Affinity Cell Selection (Fab-TACS®/Nano-TACS®) and MHC I Streptamer®.
In both principles, the key elements are capture proteins fused to a Twin-Strep-tag® and fluorescently conjugated Strep-Tactin®. For surface marker-specific staining, Twin-strep-tagged Fab fragments (Fab-Streps) or nanobodies (Nano-Streps) are used, whereas Twin-strep-tagged MHC I-peptide complexes (MHC I-Streps) are required for antigen-specific cell staining. Combining them with fluorescently conjugated Strep-Tactin® generates complexes that are suitable for cell staining.
An important feature of Fab-Streps and Nano-Streps is their low affinity. They are developed in a way that they specifically, but only weakly, bind to a target. In turn, monomers of MHC I-peptide complexes bind to the T cell receptor naturally with a low affinity. Therefore, all capture proteins (Fab-Streps, Nano-Streps and MHC I-Streps) require multimerization on a fluorescent Strep-Tactin® backbone to increase their avidity. The resulting complexes stably bind to cells.
The multimerization of capture proteins on Strep-Tactin® is reversible. Biotin has a much higher affinity to Strep-Tactin® than the Twin-Strep-tag® and causes the release of the bound capture proteins from the fluorescent Strep-Tactin® conjugate. Consequently, monomeric Fab-, Nano- or MHC I-Streps spontaneously dissociate from the cell surface, which results into completely label-free cells.
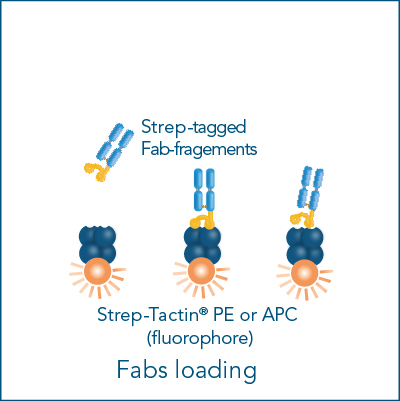
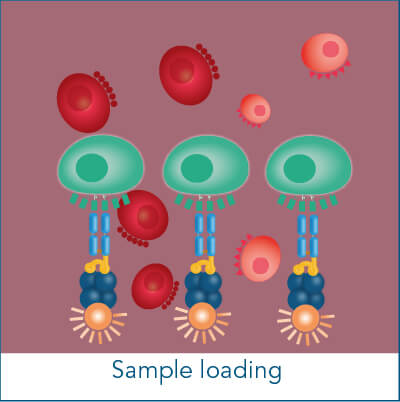
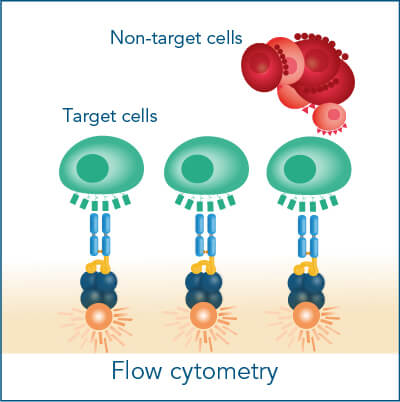
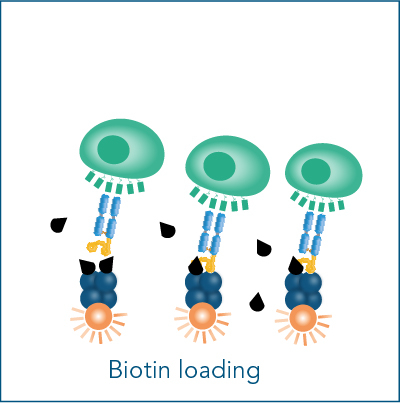
The Fab-TACS® cell staining workflow includes the following steps: Fab-Streps have to be loaded on fluorescently conjugated Strep-Tactin®. The resulting complexes are used to labels cells for subsequent flow cytometric analysis and/or cell sorting. After washing away all unwanted cells, biotin addition releases Fab-Streps from fluorescent Strep-Tactin® and causes the spontaneous dissociation of Fab-Streps from the cell surface (elution).
The Strep-tag® technology is applicable to polyclonal stimulation and expansion of T cells with CD3/CD28 Streptamer® as underlying principle. In this approach, antibody-derived Fab fragments target CD3 and CD28 on the surface of T cells. These Fab fragments are fused to a Twin-Strep-tag® (Fab-Streps), permitting multimerization of CD3 and CD28 Fab-Streps on Strep-Tactin® multimers, generating CD3/CD28 Streptamers.
The multimerization of Fab-Streps on Strep-Tactin® is necessary, because the Fab-Streps used in this approach only have a low affinity to their target. Therefore, a stable binding to T cells is only accomplished if the avidity is sufficiently increased. Only the multimerization of Fab-Streps on Strep-Tactin® generates a soluble complex that efficiently activates T cells, as monomeric Fab-Streps fail to do so.
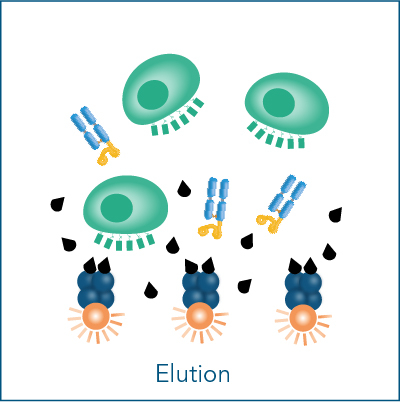
The advantage of using CD3/CD28 Streptamers is the possibility to stop the stimulation any time during the expansion period. Biotin provides this option, because it has a much higher affinity to Strep-Tactin® than the Twin-Strep-tag®. It blocks all binding pockets on Strep-Tactin® and thereby disrupts the multimerization of Fab-Streps. This terminates cell stimulation within minutes after biotin addition. Due to the low affinity of the Fab-Streps, they spontaneously dissociate from the cell surface, ending the stimulation. This facilitates highly controlled T cell activation with nearly instantaneous initiation and termination of the activation signal and offers the possibility to generate completely reagent-free cells applicable for various downstream applications.
Find out more in our application examples, in which CD3+ T cells were isolated from PBMCs and stimulated and expanded with CD3/CD28 Streptamers.
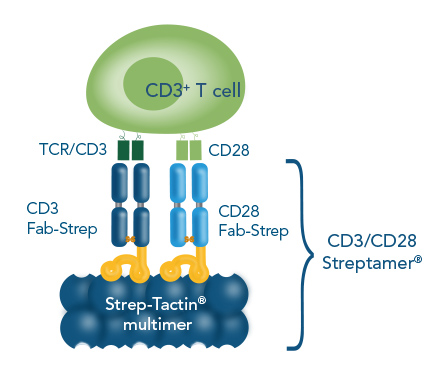
For the generation of stable T cell activation complexes, Fab fragments fused to a Twin-Strep-tag® (Fab-Streps) against CD3 and CD28 are combined with Strep-Tactin® multimers to generate soluble CD3/CD28 Streptamers.
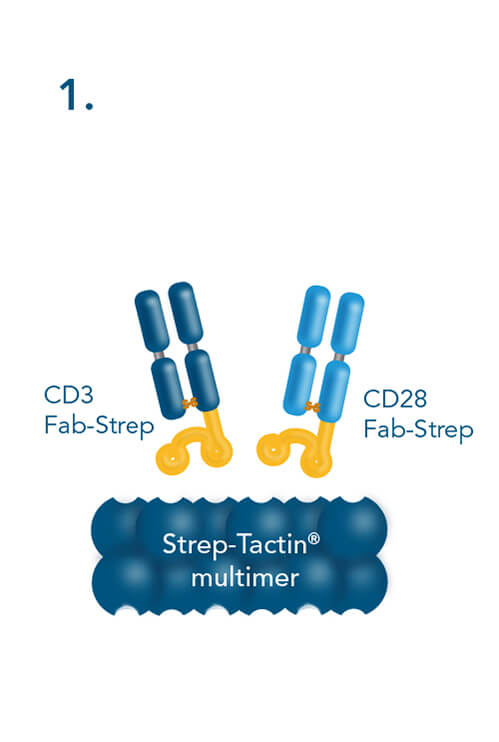
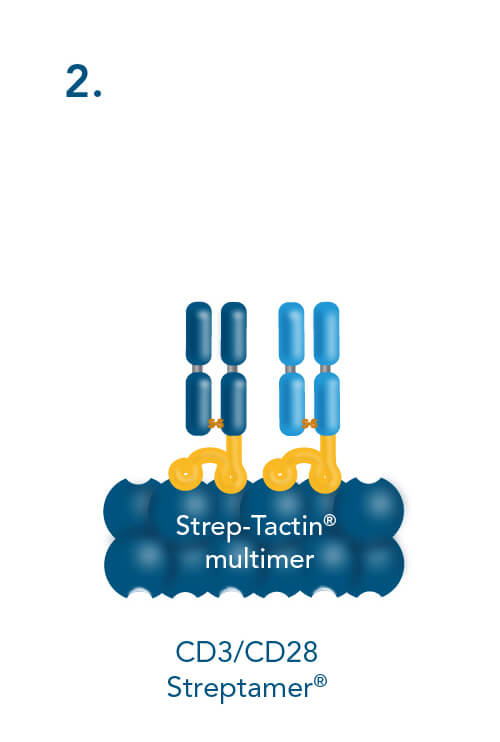
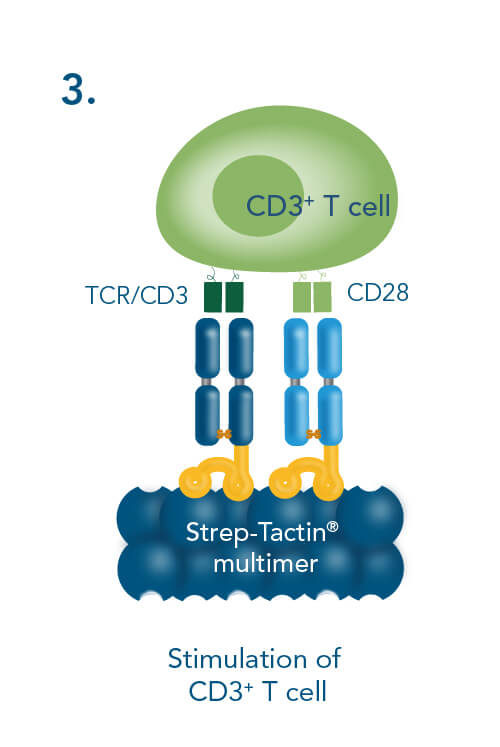
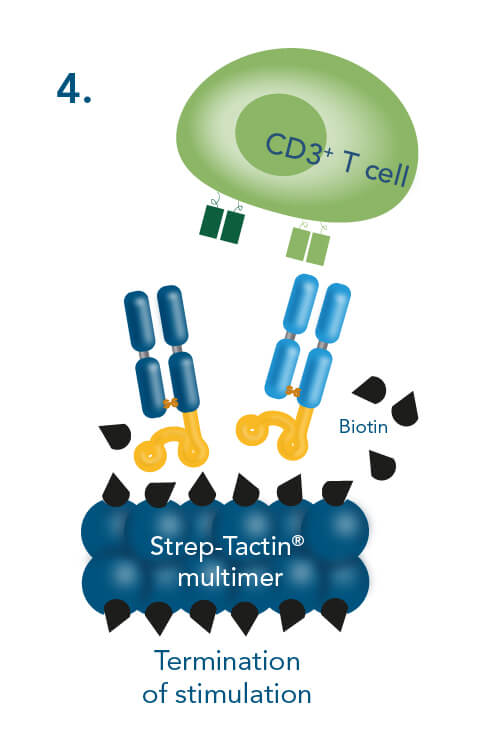
T cell stimulation & expansion using CD3/CD28 Streptamers includes the following steps: combining CD3- and CD28-Fab Streps with Strep-Tactin® multimers, adding the soluble CD3/CD28 Streptamer® complexes to cells for activation and expansion and terminating the stimulation by adding biotin.