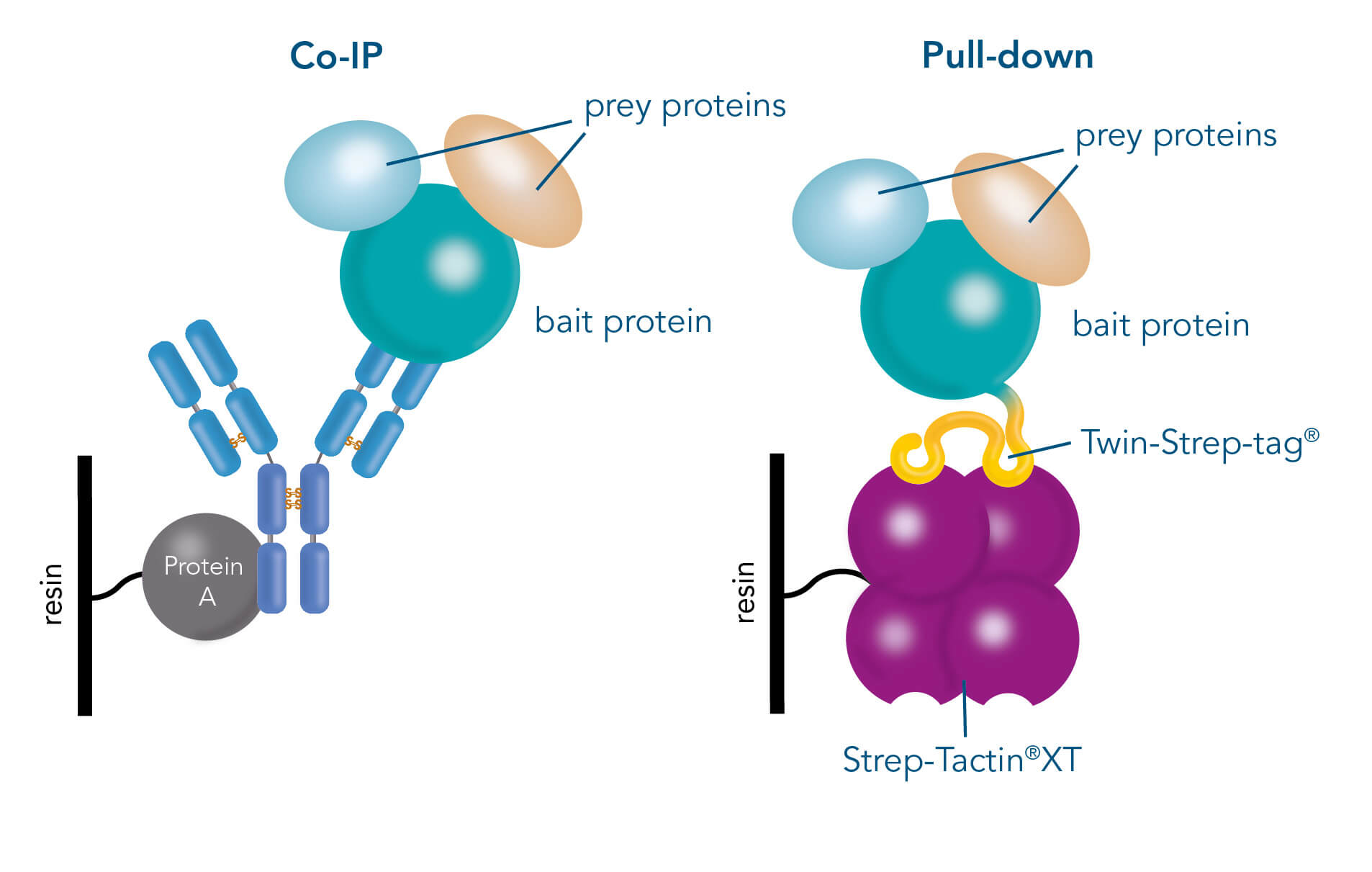
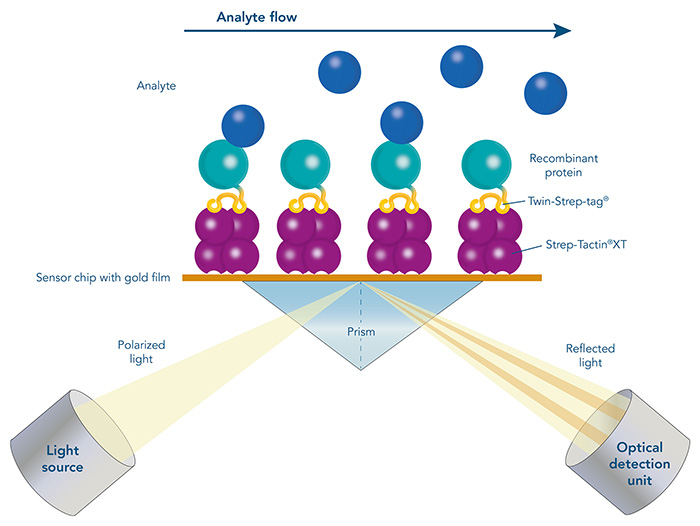
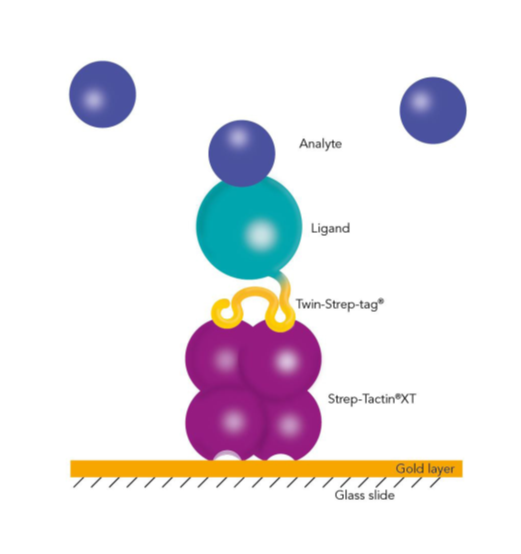
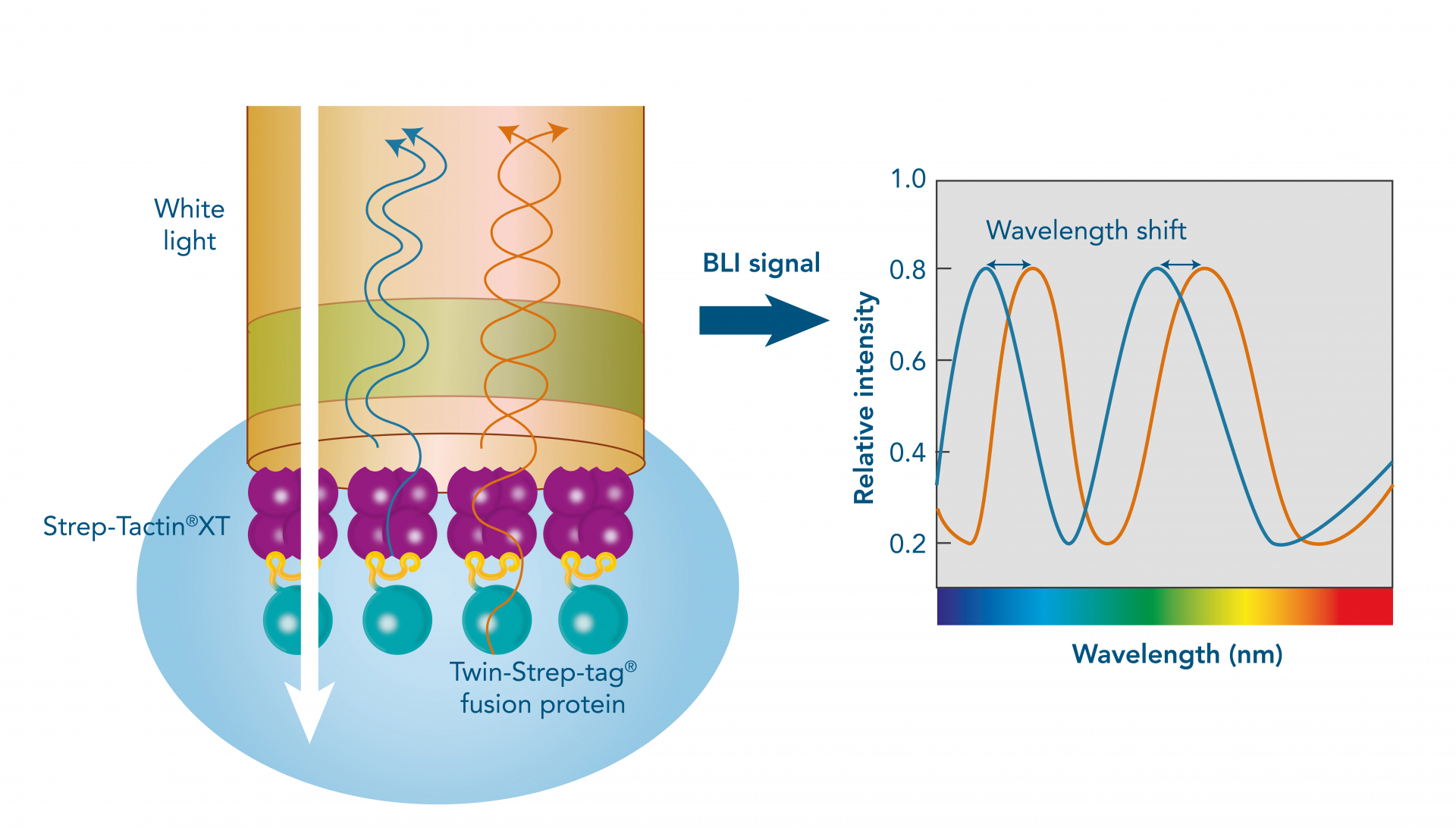
Immunoprecipitation (IP), Co-immunoprecipitation (Co-IP) and pull-down assays represent different methods to concentrate the target from a mixture for further downstream analysis such as the abundance, the activation status or the protein-binding partners.
While IP is used for capturing one specific target from a mixture, Co-IP and pull-down assays not only focus on single proteins but also on molecules which are attached to the target protein. Identifying the cellular interaction partners of a protein of interest and determining the biological pathways involved is a necessary preliminary work to gain deeper insights into a target’s structure and function. When it comes to the investigation, IP, Co-IP and pull-down assays have become invaluable tools, which are built upon the affinity-based immobilization of a “bait” protein on a solid support, mostly magnetic or agarose beads.