Research on SARS-CoV-2 is currently a key concern of life sciences to prevent further infections and to develop a vaccine. The following studies as well as the antigen specific FluoroSpot method demonstrate the great utility of the Strep-tag® technology in this area. It can be used for the isolation of cells and exosomes or for affinity purification of proteins, but moreover Strep-Tactin®XT proves to be particularly good for the immobilization and detection of viral proteins due to its high affinity for the Twin-Strep-tag®.
IBA COVID-19
Research Solutions for Coronavirus
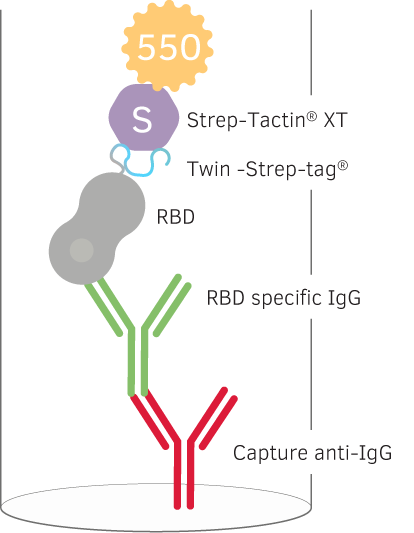
Detection of Memory B cells specific for SARS-CoV-2 with reversed antigen specific IgG FluoroSpot
The Swedish biotech company Mabtech showed that reversed antigen specific FluoroSpot is a valid method for testing human B cell memory response, which could be an interesting complement for studying long term immunity of COVID-19.
In their study they immobilized their anti-human IgG (MT91/145) capture antibodies in a FluoroSpot plate. For detection of IgGs specific to a receptor binding protein (RBD) of the SARS-CoV-2 Spike protein, the RBD was fused with a C-terminal Twin-Strep-tag® that subsequently was detected with a Strep-Tactin® XT conjugated to a 550-fluorophore. They decided to recombinantly express the Twin-Strep-tag® together with the RBD protein instead of traditional biotinylation, because biotinylation of lysines might interfere with immunogenic epitopes. More information about the method can be found here.
Our tools for COVID-19 research
| Catalogue number | Product |
| 2-1502-001 | Strep-Tactin® HRP Conjugate |
| 2-1562-050 | Strep-Tactin®XT DY-488 conjugate |
| 2-1568-050 | Strep-Tactin®XT DY-649 conjugate |
| 2-1507-001 | StrepMAB-Classic, unconjugated |
| 2-1509-001 | StrepMAB-Classic, HRP conjugate |
| 6-5000-001 | Strep-Tactin® PE for Streptamers |
| 6-5010-001 | Strep-Tactin® APC for Streptamers |
| MHC I Streptamer® Custom Service |
| Catalogue number | Product |
| 6-3203-004 | CD8 Fab-TACS® Isolation Kit, human |
| 6-3203-010 | CD8 Fab-TACS® Isolation Kit, human |
| 6-3213-004 | CD19 Fab-TACS® Isolation Kit, human |
| 6-3213-010 | CD19 Fab-TACS® Isolation Kit, human |
| Catalogue number | Product |
| 6-3219-004 | CD9 Fab-TACS® Exosome Isolation Kit, human |
| 6-3219-010 | CD9 Fab-TACS® Exosome Isolation Kit, human |
| 6-3281-004 | CD81 Fab-TACS® Exosome Isolation Kit, human |
| 6-3281-010 | CD81 Fab-TACS® Exosome Isolation Kit, human |
| 6-3295-004 | CD9/CD81 Fab-TACS® Exosome Isolation Starter Kit, human |
| 6-3296-002 | CD9 Fab-TACS® Exosome Isolation Introductory Kit, human |
| 6-3297-002 | CD81 Fab-TACS® Exosome Isolation Introductory Kit, human |
| Catalogue number | Product |
| 6-7111-901 | SARS-CoV-2 peptide FIAGLIAIV (HLA-A*0201) |
| 6-7112-901 | SARS-CoV-2 peptide ALNTLVKQL (HLA-A*0201) |
| 6-7113-901 | SARS-CoV-2 peptide VLNDILSRL (HLA-A*0201) |
| 6-7114-901 | SARS-CoV-2 peptide VVFLHVTYV (HLA-A*0201) |
| 6-7115-901 | SARS-CoV-2 peptide ILLNKHIDA (HLA-A*0201) |
| 6-7116-901 | SARS-CoV-2 peptide LALLLLDRL (HLA-A*0201) |
| 6-7117-901 | SARS-CoV-2 peptide LLLDRLNQL (HLA-A*0201) |
| 6-7118-901 | SARS-CoV-2 peptide LQLPQGTTL (HLA-A*0201) |
| 6-7119-901 | SARS-CoV-2 peptide KIADYNYKL (HLA-A*0201) |
| 6-7120-901 | SARS-CoV-2 peptide KVGGNYNYL (HLA-A*0201) |
| 6-7121-901 | SARS-CoV-2 peptide GYQPYRVVVL (HLA-A*2402) |
| 6-7122-901 | SARS-CoV-2 peptide PYRVVVLSF (HLA-A*2402) |
| 6-7123-901 | SARS-CoV-2 peptide LSPRWYFYY (HLA-A*2402) |
| 6-7124-901 | SARS-CoV-2 peptide FVSEETGTL (HLA-A*0206) |
| Catalogue number | Product |
| 2-1201-010 | Strep-Tactin® Sepharose® |
| 2-1206-025 | Strep-Tactin® Superflow® |
| 2-4021-001 | Strep-Tactin®XT Superflow® cartridge |
| 2-4026-001 | Strep-Tactin®XT Superflow® high capacity cartridge |
| 2-4090-002 | MagStrep “type3” beads |
| 6-2010-005 | Protein A Agarose |
| Catalogue number | Product |
| On request | Strep-Tactin® |
| On request | Strep-Tactin®XT |
| Catalogue number | Product |
| 2-0205-050 | BioLock (Biotin blocking solution) |
| 2-1042-025 | Strep-Tactin®XT Elution Buffer (10x Buffer BXT) |
| 2-1000-002 | Desthiobiotin |
| 2-1016-005 | Biotin |
| Catalogue number | Product |
| 2-0911-001 | WET FRED for gravity flow columns |
More relevant papers for COVID-19 research
A SARS-CoV-2 protein interaction map reveals targets for drug repurposing
Gordon et al., made great progress in finding therapies that may reduce the mortality of COVID-19. The researchers identified the human proteins that associate with viral proteins of SARS-CoV-2 using an affinity-purification mass spectrometry approach. In this regard, they expressed Twin-Strep-tagged versions of SARS-CoV-2 proteins in human cells, which they could verify by anti-Strep western blot. Next, Gordon and colleagues purified the expressed Twin-Strep-tagged viral proteins together with associated human proteins using MagStrep “type3” XT beads. After elution of the viral proteins with buffer BXT, they analyzed the co-eluted proteins by mass spectrometry and could reveal 332 human proteins that with high confidence interact with SARS-CoV-2. Out of these proteins, 66 can be targeted by existing drugs that are either approved, in clinical or pre-clinical stages. In a first screen for their anti-viral properties, two sets of pharmacological agents seem promising, inhibitors of mRNA translation and predicted regulators of the Sigma1 and Sigma2 receptors. Although further studies of the potential therapeutics for efficacy in SARS-CoV-2 infection are required, the run towards a COVID-19 treatment might be accelerated.
For detailed information read the full paper here.
Gordon, D.E., Jang, G.M., Bouhaddou, M. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature (2020).
Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2
To infect a host cell, the Coronavirus Sars-CoV-2 must bind to the receptor protein angiotensin-converting enzyme 2 (ACE2) via its surface spike proteins. Yan et al. were able to reveal the structure of the human ACE2 with and without the binding domain of the SARS-CoV-2 spike protein. They found that ACE2, which was formerly difficult to measure, could be stabilized by the presence of the membrane protein B0AT1. Thus, the authors co-expressed Strep- and FLAG-tagged B0AT1 together with ACE2 in a human cell line. Then they purified the protein complex through a tandem affinity approach using anti-FLAG affinity resin and Strep-Tactin® Sepharose® followed by size exclusion chromatography. After mixing a part of the purified samples with the SARS-CoV-2 binding domains, the resulting complexes were studied by cryo–electron microscopy. The researchers could present the structure of ACE2 forming a dimer which is enclosed by B0AT1. A further structure with the bound SARS-CoV-2 spike binding domain suggests that two virus spike proteins bind to one ACE2 dimer. The remarkable findings of Yan and colleagues could be helpful for the development of therapeutics that interfere with the virus-host cell interaction and thus prevent infection.
For detailed information read the full paper here.
Yan, R., Zhang, Y., Li, Y., Xia, L., Guo, Y., & Zhou, Q. (2020). Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science, 367(6485)
Site-specific glycan analysis of the SARS-CoV-2 spike
The spike (S) proteins of SARS-CoV-2 that mediate membrane fusion and host cell entry, contain several host-derived sugars, so called glycans. These glycoproteins provide fundamental features of viral biology, as they play key roles in protein folding and immune evasion. Watanabe and colleagues could reveal the glycan structures of a recombinant SARS-CoV-2 S protein. The researchers expressed Twin-Strep-tagged versions of the SARS-CoV-2 S protein in HEK cells and purified them from cell supernatants using Strep-Tactin®. Then they combined mass spectrometric and cryo-EM analyses to determine how the glycans cover distinct regions of the virus spike surface. With this site-specific glycan signature of the SARS-CoV-2 spike, the research team improved the understanding of viral biology with major implications for vaccine development.
For detailed information read the full paper here.
Watanabe, Yasunori & Allen, Joel & Wrapp, Daniel & Mclellan, Jason & Crispin, Max. (2020). Site-specific glycan analysis of the SARS-CoV-2 spike. Science. eabb9983.
A human monoclonal antibody blocking SARS-CoV-2 infection
Monoclonal antibodies that target viral surface proteins are increasingly recognized as promising class of drugs for treating infectious diseases. Currently, Wang and colleagues discovered an antibody that binds to SARS-CoV2 and SARS-CoV by using an ELISA cross reactivity approach. They tested a collection of antibody-containing hybridoma supernatants that were obtained from SARS-CoV immunized transgenic mice. One antibody was able to bind to spike proteins from both viruses, SARS-CoV2 and SARS-CoV. This chimeric antibody was reformatted and expressed as a full human IgG1 isotype. Further experiments revealed that the antibody can neutralize infection of VeroE6 cells with SARS-CoV2 and SARS-CoV. Although the mechanism that mediates virus neutralization is not clear yet, a promising first step towards COVID-19 diagnostic, prevention and treatment was taken.
In the study, Strep-tagged spike ectodomains of SARS-CoV2 and SARS-CoV were expressed in HEK-293T cells and affinity purified with Strep-Tactin beads. In the ELISA experiments, the equimolar coating of the Strep-tagged spike antigens was controlled by using the HRP-conjugated anti StrepMAB antibody.
For detailed information read the full paper here.
Wang, C., Li, W., Drabek, D. et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 11, 2251 (2020).
Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19
Many of the severe cases of the currently spreading COVID-19 result from an over-reaction of the immune system. Exosomes derived from mesenchymal stem cells (MSCs) have the potential for an effective treatment of immune-mediated diseases, because they constitute the strong immunosuppressive effects of MSCs, while overcoming their limitations in availability, safety, and stability. In a prospective nonrandomized open-label study, Sengupta et al. explored the efficacy and safety of exosomes (ExoFlo™) derived from bone marrow MSCs in the treatment of severe COVID-19. They provided ExoFlo™ intravenously to 24 SARS-CoV-2 positive patients with severe symptoms. After the treatment, patient’s oxygenation and inflammatory markers improved significantly, while no adverse events occurred within the observation period. Although further randomized controlled trials are required, the possible application of exosomes as a therapeutic against severe COVID-19 seems encouraging.
Read more about our novel method for a quick and simple isolation of pure and functional exosomes here.
For detailed information read the full paper here.
Sengupta, Vikram & Sengupta, Sascha & Lazo, Jr & Woods, Peter & Nolan, Anna & Bremer, Nicholas. (2020). Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells and Development.
