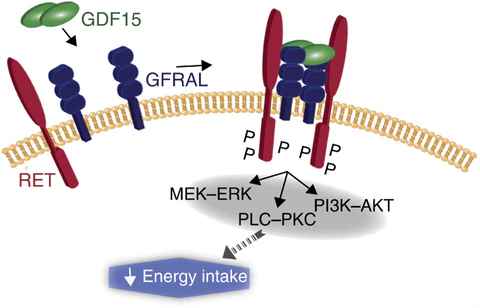
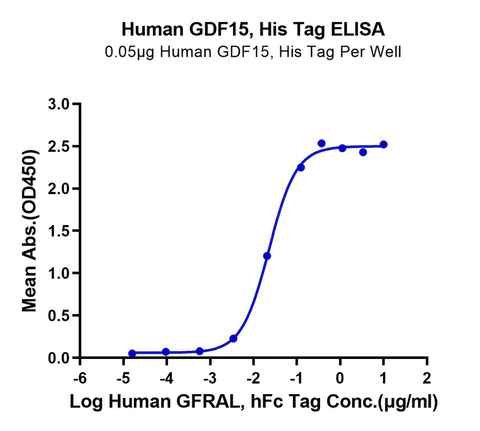
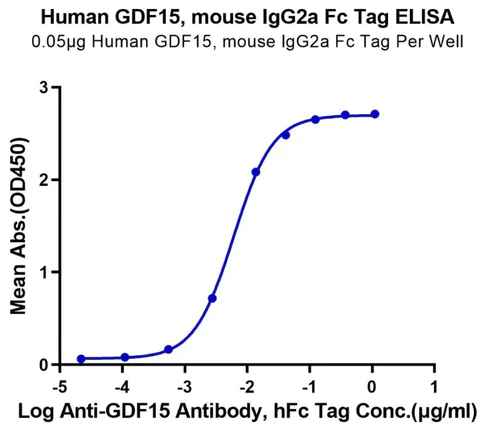
A variety of metabolic diseases that plague today’s society, such as obesity, diabetes, cancer, etc., have yet to be solved. Several major studies have confirmed that the GDF15-GFRAL pathway is involved in regulating the body’s metabolism, inflammation, and immune response. This suggests that GDF15-GFRAL can be a target for the treatment of related diseases.
GDF15
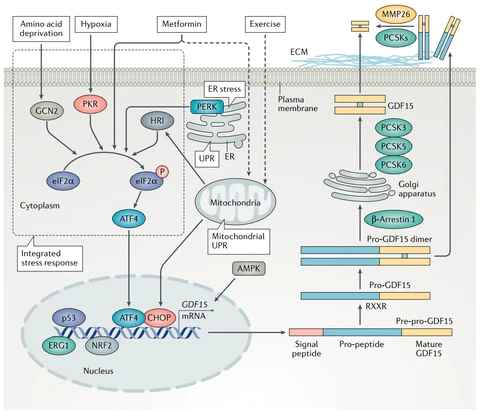
GDF15 (Growth Differentiation Factor 15) belongs to the TGFβ (Transforming growth factor β) superfamily. The two proteins of GDF15 first form a dimer structure through a disulfide bond, which is then hydrolyzed at the RXXR site to form the mature form of GDF15. This form is then secreted outside the cell. In some cells, the precursor dimer can also be secreted separately and attach to the extracellular matrix until cleaved (Figure 1).
GDF15 is an endocrine hormone mainly involved in cell growth, differentiation, and tissue repair. Under normal physiological conditions, GDF15 is highly expressed in the prostate and placenta, and is weakly expressed in most tissues such as the heart. However, it can be up-regulated when subjected to external stress (such as hypoxia, mitochondrial damage, metformin, and endurance exercise) (Figure 1). This feature gives GDF15 important auxiliary diagnosis or treatment value in myocardial infarction, stroke, and acute coronary syndrome.
GDF15, first discovered in 1997 [1], acts as an autoregulatory factor in macrophages that inhibits lipopolysaccharide-induced tumor necrosis factor-alpha (TNFα) release. Due to its discovery by different researchers, GDF15 has been given different names: TGF-PL, MIC-1 (Macrophage Inhibitory cytokine-1), PDF (Prostate-derived factor), PLAB (Placental bone morphogenetic protein), NAG-1 ( Non-steroidal anti-inflammatory drug-inducible gene), PTGFB (Placental transforming growth factor-beta).