Nanoparticles have many applications in biology and medicine due to their unique optical and physical properties. These versatile reagents can be used for biosensor development, as cellular probes, as drug delivery vehicles, or as optical contrast agents among others.
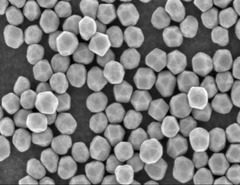
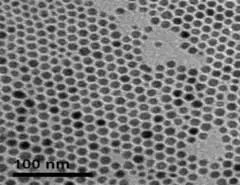
Cytodiagnostics spherical and non-spherical nanoparticle products are made with proprietary protocols resulting in particles with uniform shapes and narrow size distributions.
By precisely engineering the nanoparticle surface, we also offer secondary antibody conjugates and particles with functional groups such as NHS, biotin, carboxyl, amine and methyl allowing them to be directly used in many applications.