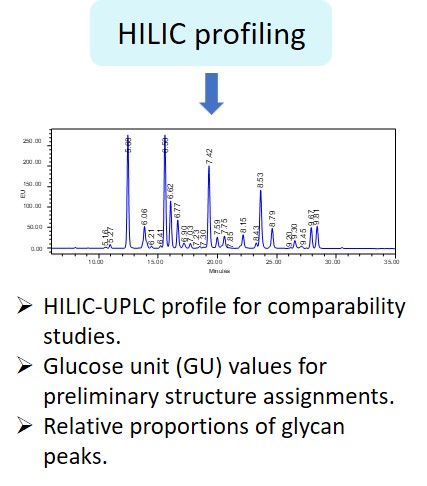
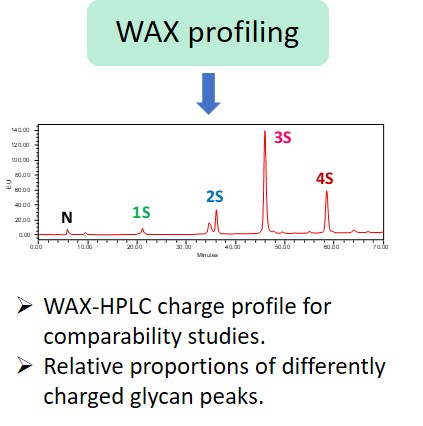
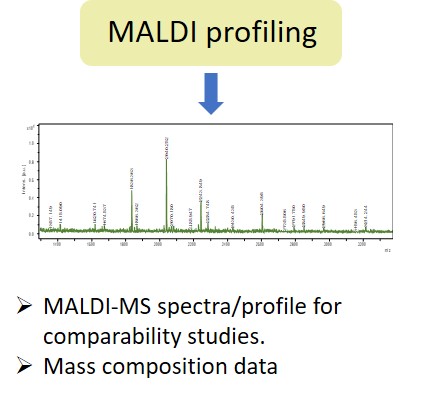
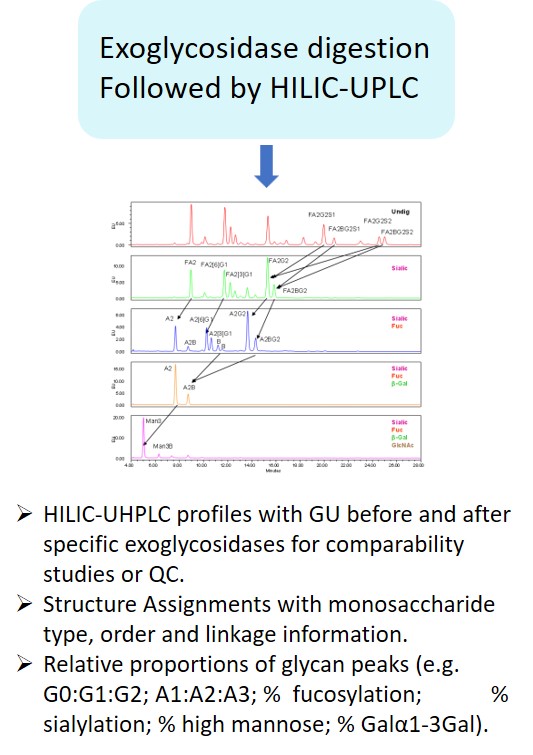
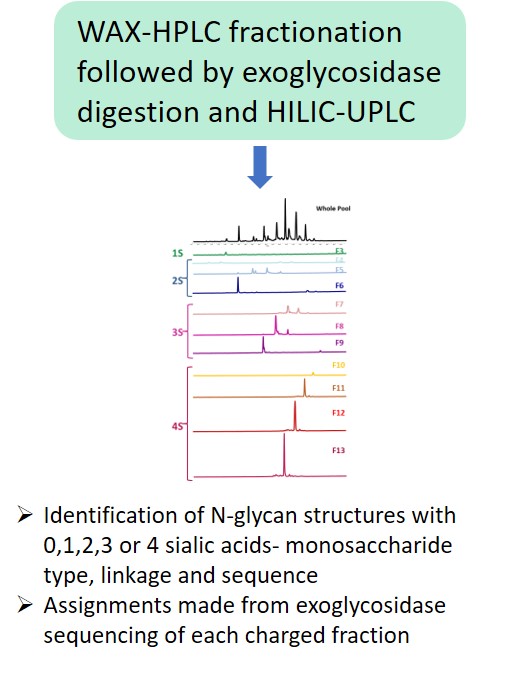
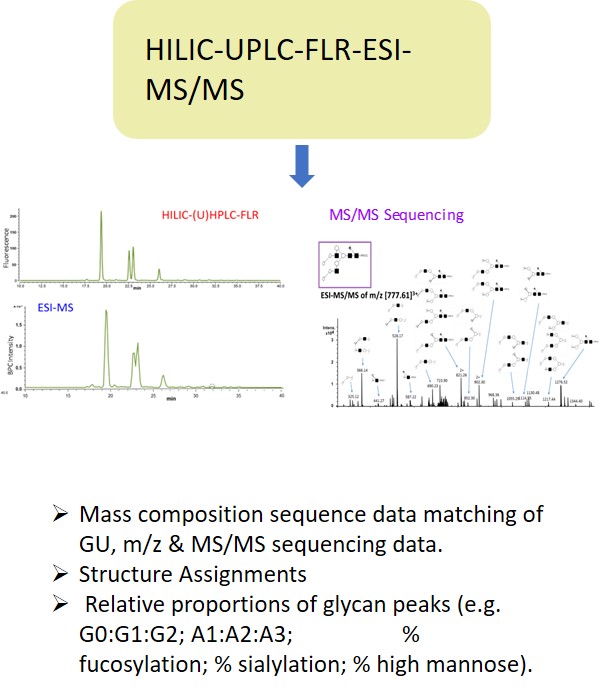
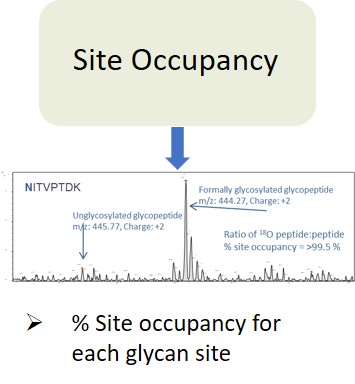
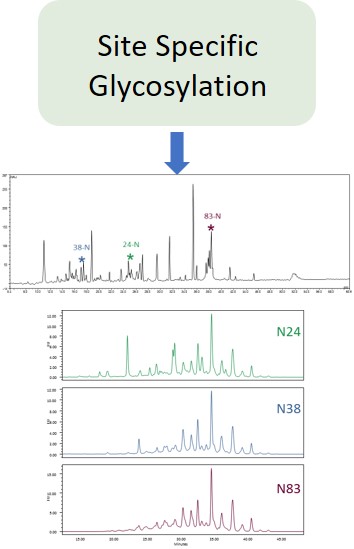
EMA guideline on development, production, characterisation and specification for monoclonal antibodies and related products states:
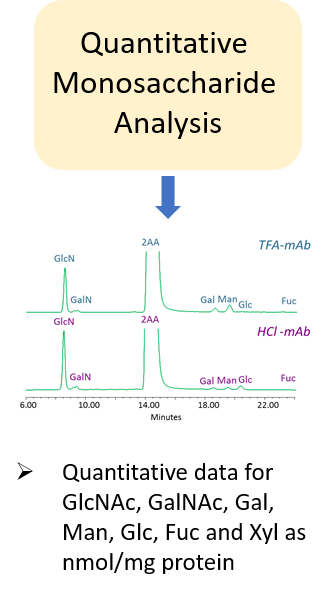
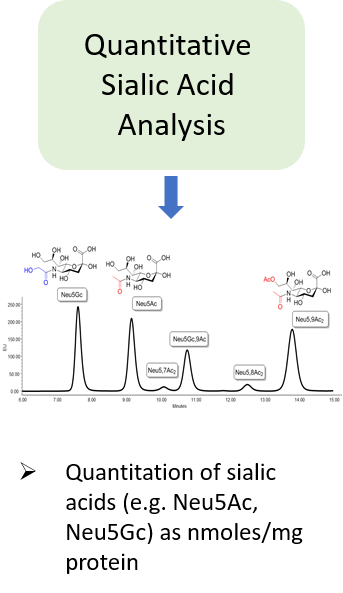
“The carbohydrate content (neutral sugars, amino sugars and sialic acids) should be determined. In addition, the structure of the carbohydrate chains, the oligosaccharide pattern (antennary profile), the glycosylation site(s) and occupancy should be analysed.
Typically, monoclonal antibodies have one N-glycosylation site on each heavy chain located in the Fc region. The light chain is usually not glycosylated. However, additional glycosylation site(s) in the heavy chains may occur, and thus their presence or absence should be confirmed. Glycan structures should be characterised, and particular attention should be paid to their degree of mannosylation, galactosylation, fucosylation and sialylation. The distribution of the main glycan structures present (often G0, G1 and G2) should be determined.
Higher-order structure of the monoclonal antibody should be characterised by appropriate physicochemical methodologies.”
“For glycoproteins, the carbohydrate content (neutral sugars, amino sugars, and sialic acids) is determined. In addition, the structure of the carbohydrate chains, the oligosaccharide pattern (antennary profile), and the glycosylation site(s) of the polypeptide chain are analyzed, to the extent possible.”
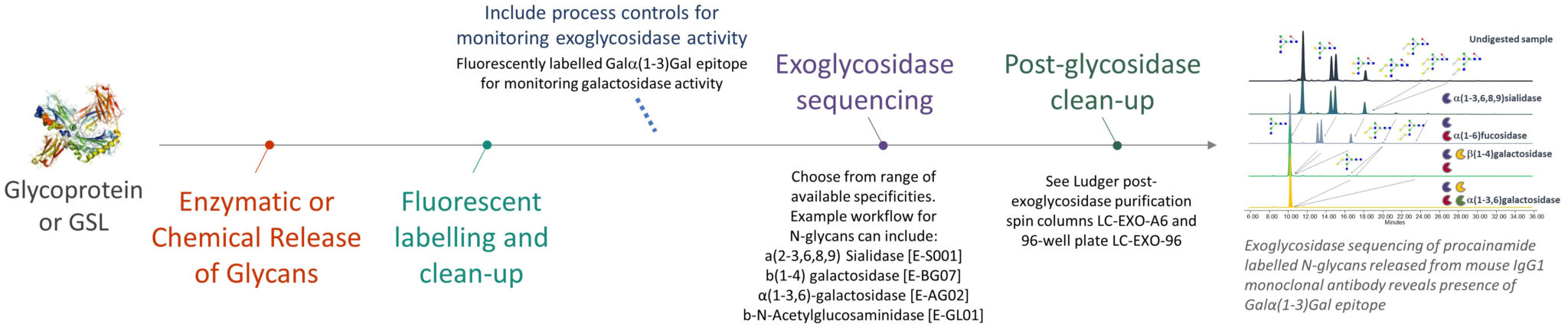
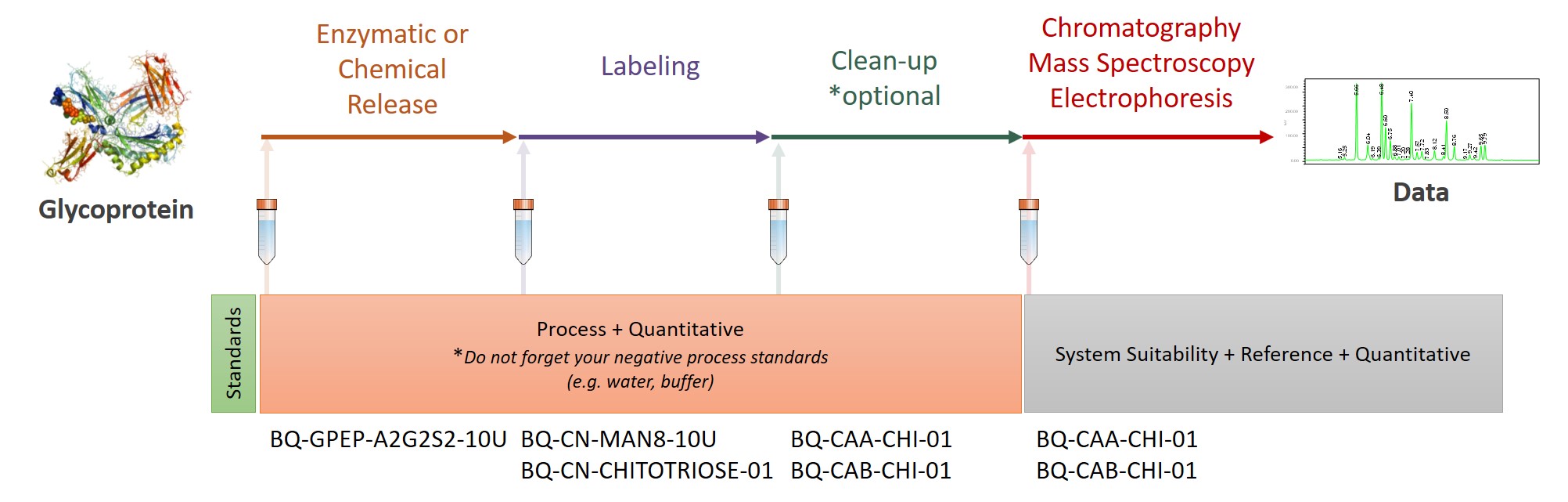
At Ludger we use a systematic approach aligned with current regulatory guidelines to support drug developers and researchers. Our system has three broad steps: