Applications
Common Applications of Secondary Antibodies
Detection Methods
Colorimetric
Reporter enzyme conjugates alkaline phosphatase (AP) and horseradish peroxidase (HRP) can be used for colorimetric detection. The conjugated reporter enzyme catalyzes the conversion of the chromogenic substrate to a colored precipitate, which is visualized directly on the blotting membrane. Colorimetric detection can offer quick and easily obtained results without the need for expensive detectors or extensive optimization.
Chemiluminescent
Enzyme-linked conjugates can also be used for chemiluminescent signal detection. HRP conjugates produce signal by oxidizing a chemiluminescent substrate (luminol) to a form which emits light. AP conjugates produce signal when the enzyme dephosphorylates a specific substrate (e.g. 1,2-dioxetane) to a light emitting product. The signal can then be captured by exposing photographic film to the membrane or using a cooled charge-coupled device (CCD) camera. Chemiluminescent detection offers excellent sensitivity, however quantification and probing for multiple targets can be limited, and development may require refinement to optimize signal capture.
Fluorescent
Fluorescent detection uses a fluorescent dye conjugate to visualize antigen on the membrane. Light at a wavelength specific to the dye’s spectral characteristic is absorbed, exciting the dye’s electrons to a higher electronic state, and as they return to their ground state they emit photons at the emission wavelength characteristic to the fluorophore. The light emitted is detected by a digital imager fitted with appropriate filters. Fluorescent Western blotting allows for quantitative analysis and multiplex probing without the need for stripping and reblotting. Jackson ImmunoResearch offers a range of fluorescent dyes covering the spectrum.
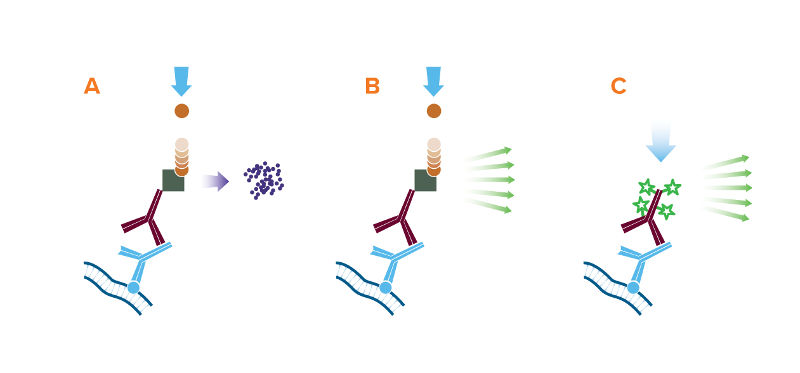
The 3 detection methods for Western blot: (A) Colorimetric, (B) Chemiluminescent, and (C) Fluorescent;
A. The reporter enzyme conjugate catalyzes the conversion of a chromogenic substrate to a colored insoluble precipitate, visible by eye on the blotting membrane.
B. The reporter enzyme conjugate catalyzes a reaction which converts the chemiluminescent substrate to a light emitting form, and the emitted light is detected by X-ray film or CCD camera.
C. The reporter fluorescent dye is excited by its characteristic wavelength light, and resulting emitted light is captured by a digital imager.
Jackson ImmunoResearch produces the largest diversity of species specific secondary antibody conjugates for use in Western blotting. JIR offers antibodies and streptavidin conjugated with horseradish peroxidase (HRP), alkaline phosphatase and Biotin-SP for use in traditional blots developed with chromogenic or chemiluminescent (ECL) substrates. In addition, secondary antibodies conjugated with fluorescent dyes, including far-red and infrared-emitting dyes, are available for multicolor imaging in modern readers.
Jackson ImmunoResearch also offers affinity-purified anti-horseradish peroxidase which may be used to detect HRP or to enhance signal by binding to HRP-conjugated molecules. Conjugated anti-HRP may be used to convert an HRP conjugate into a different signal.
Western blotting after Immunoprecipitation (IP)
Probing in the 50 kDa range after IP
If diluted properly, anti-light chain specific antibodies do not bind to the reduced and denatured IgG heavy chain band (50 kDa) on blots. Therefore, by using anti-light chain specific antibodies, detection of antigens with molecular weights near 50 kDa is not obscured by large amounts of reduced and denatured IgG heavy chains from primary antibodies used for immunoprecipitation (IP).
Caution: Although the antibodies react strongly with native IgG light chains, some do not react as strongly with reduced and denatured light chains on blots. Therefore, they are not recommended for sensitive and quantitative detection of reduced and denatured light chains.
Probing in the 25 kDa range after IP
If the protein of interest (POI) has a reduced and denatured molecular weight near 25 kDa, anti-IgG, Fc fragment specific antibodies may be used to detect IgG primary antibodies, without binding to reduced and denatured IgG light chains on Western blots. Clear detection of a POI near 25 kDa requires blocking degraded heavy chain that migrates to 25 kDa.
Near-Infrared (NIR) fluorescent conjugates
Antibodies conjugated with far-red- and infrared-emitting dyes are suitable for a variety of techniques requiring the highest sensitivity. Alexa Fluor® 680 and 790 dyes are more sensitive than visible light-emitting dyes due to lower fluorescence quenching of the conjugates and higher extinction coefficients of the dyes. Low sample autofluorescence in this region of the spectrum results in lower background signal compared with other fluorophores.
Alexa Fluor® 680 and 790 conjugates can be used for high sensitivity Western blots, quantitative Western blots, in-gel Western blots, microWestern arrays, in-cell Western assays, on-cell Western assays, and other techniques that require the brightest dyes.
Usage Guidance
Membrane blocking buffer
Normal serum from the host species of the labeled antibody (5% v/v) is an excellent block, although 5% non-fat milk and 3% BSA are commonly used and may also be effective. Avoid milk or BSA when using a primary antibody derived from goat or sheep.
Diluting antibodies for Western blotting
PBS/Tween 20 (0.05% v/v) or TBS/Tween, without carrier proteins, is recommended as the secondary antibody diluent. Especially when using anti-goat or anti-sheep secondary antibodies, avoid using milk or BSA in the diluent buffer. Bovine IgG in the milk or BSA may interact with the antibody due to homologous epitopes of the related species.
Recommended dilution ranges for our conjugated secondaries in Western blotting are shown in the tables below.
| Product | Chemiluminescent | Chromogenic |
| HRP-Antibody | 1:10K - 1:200K | 1:5K - 1:100K |
| Alkaline Phosphatase-Antibody | 1:5K - 1:50K | 1:5K - 1:50K |
| Biotin-Antibody | 1:20K - 1:400K | 1:20K - 1:400K |
| HRP-Streptavidin | 0.01 - 0.1 μg/mL | 1 - 2 μg/mL |
| Alkaline Phosphatase-Streptavidin | 0.1 - 1.0 μg/mL | 1 - 2 μg/mL |
| PAP | 1:5K - 1:100K | 1:5K - 1:100K |
| Product | Fluorescent |
| Alexa Fluor® 488, 594, 647, DyLight™ 405, and Cy™3-Antibody | 1:100 - 1:800 |
| AMCA, BV421™, BV480™, Cy2, FITC, TRITC, RRX, and Texas Red-Antibody | 1:50 - 1:200 |
| Alexa Fluor® 680 and 790-Antibody | 1:50K - 1:200K |
| Alexa Fluors®, DyLight 405, Cy3, and Cy5-Streptavidin | 0.5 - 2 µg/ml |
| Other fluorophores-Streptavidin | 2 - 5 µg/ml |
IHC with JIR Secondary Antibodies
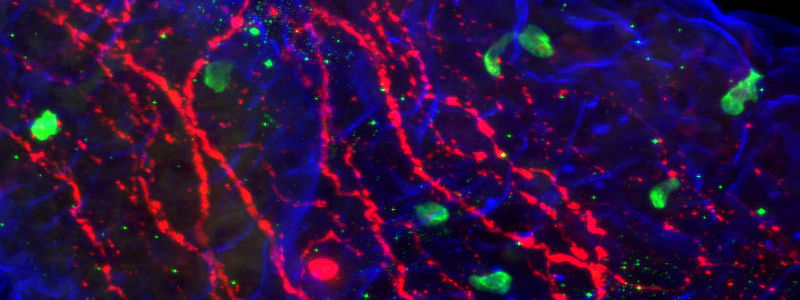
Figure 1: Triple immunofluorescence. Jejunum villus probed for Ulex – Biotin using Cy™5 Streptavidin (blue 016-170-084), GIP by Cy™2 AffiniPure Donkey Anti-Goat IgG (H+L) (green 705-225-147), tubulin by Cy™3 AffiniPure Donkey Anti-Mouse IgG (H+L) (red 715-165-150). Image courtesy of Brian McAdams and William Kennedy, University of Minnesota.
Immunohistochemistry (IHC) is a powerful technique, indispensable in research and in clinical diagnostics. It is a staple of the pathology lab for disease diagnostics and classification. In research, IHC is used to interrogate many biological processes, including visualization of protein expression patterns, characterization of protein interactions, and identification of tissue boundaries. The researcher can thereby observe the distribution and localization of specific structures within the context of the cellular architecture.
Principles of Immunohistochemistry
A tissue analyte is specifically recognized by a primary antibody, which may be directly conjugated (direct IHC); or the primary may itself be detected by a conjugated secondary antibody (indirect IHC), allowing visualization of the analyte. The conjugated antibody can be labeled with any of several reporter molecules, including enzymes, fluorophores and colloidal gold. The expression of multiple analytes can be observed and characterized by individual primary/secondary antibody pairs in multiple labeling protocols.
Direct or Indirect Immunohistochemistry
Analytes of interest may be detected on the surface of the tissue or interrogated internally through permeabilization of the sample. The analyte (antigen) can be detected directly (Figure 2 A), or indirectly (Figure 2 B) using a fluorescent (immunofluorescence) or reporter enzyme (colorimetric) probe conjugated to a secondary antibody.
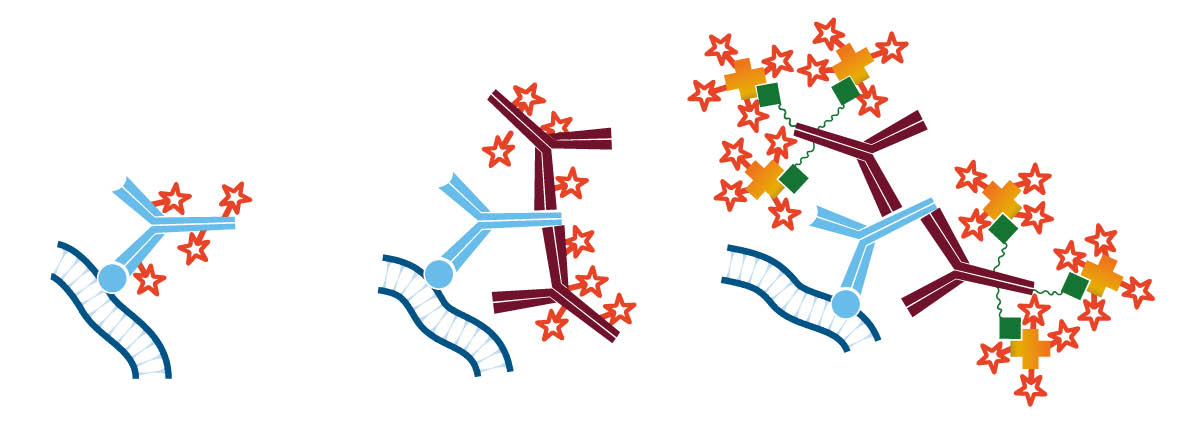
The indirect method using a secondary antibody offers many advantages. It preserves the antigen binding site on the primary antibody by conjugating to the secondary antibody. Since secondary antibodies are available conjugated to a wide variety of fluorophores, the indirect method enhances the possibilities for multiple labeling. In addition, it provides inherent signal enhancement, whereby multiple secondary antibodies bind to one antigen-bound primary antibody. Signal can be further enhanced by using a biotinylated secondary antibody followed by conjugated streptavidin (Figure 2 C), or by using the peroxidase-anti-peroxidase (PAP) method.
Designing the indirect IHC protocol
Factors that contribute to the success of IHC experiments are the tissue of interest, primary antibody choice, secondary antibody specificity, and options for signal detection. Proper blocking assures optimal signal to noise ratio, and control proteins aid in the interpretation of results.
Tissue or sample specifics
Tissue Species
The source (species) of experimental sample will influence the choice of antibodies. Certain tissue types contain endogenous immunoglobulin (Ig), and others exhibit vasculature in which remnant blood may provide endogenous Ig. For indirect IHC, the secondary antibody must recognize the specific primary antibody but not endogenous Ig. It is most convenient to use a primary antibody that is derived from a different host animal than the tissue of interest: in this case select a secondary antibody that has been cross-adsorbed (min X) to minimize recognition of endogenous Ig. If the experimental strategy includes using a primary antibody from the same host species as the tissue of interest, endogenous Ig can be masked by blocking with monovalent Fab fragments of secondary antibodies. It is also possible to label primary antibodies with Fab fragments prior to IHC incubation.
Read more about Fab fragment secondary antibodies
Fixation
Tissue fixation is the process of stabilizing and preserving a specimen in a configuration that approximates its natural state. As the innate aqueous environment is altered by chemical fixation, an antigenic site may be modified so that it cannot be recognized by a primary antibody that would detect its native state. Many primary antibodies are marketed as “validated” to perform under specified fixation conditions, though this information may not be available. In some cases, antigen retrieval protocols can return antigenic sites to their native state.
Note that the concept of antibodies validated to perform in IHC is unique to primary antibodies. If a primary antibody binds its target site, a labeled secondary antibody will bind to it regardless of tissue fixation.
Intrinsic signal
Tissue samples may reveal several types of intrinsic signal. Autofluorescence (signal in the absence of fluorescent probe molecules) may be evident in some regions of the spectrum. When planning an immunofluorescent protocol, observe an unlabeled tissue sample using available filter sets. Choose fluorophores that are compatible with spectral regions that have limited autofluorescence. It is possible to suppress autofluorescence with various reagents, though the efficiency of these treatments varies depending on both tissue type and fixation method.
Intrinsic signal may also derive from endogenous enzymes and/or biotin. To test for and control these types of background signal, see our Blocking and Controls guide.
Primary antibody choice for IHC
Target
The primary antibody should recognize the protein of interest under the intended experimental conditions. Some primary antibodies have been validated for use under specific conditions, e.g. fixation methods. An antibody that has been validated only for Western blotting may not be appropriate for IHC.
Monoclonal or polyclonal
Polyclonal antibodies recognize multiple epitopes, maximizing the opportunity for the antibody to detect the antigen, but may result in detection of homologous proteins and lead to background signal. Monoclonal antibodies consist of identical immunoglobulin molecules produced from a single cell line, and are appropriate when the detection needs are epitope specific. However, they may give a weaker signal than polyclonals. Controls can be used to help determine the suitability of antibodies for each assay system.
Host species of the primary antibody
Ideally, the host species of the primary antibody should be a different species from the sample species to avoid cross-reactivity with endogenous proteins. If the primary antibody and the tissue of interest are the same species, blocking protocols can be used to prevent off-target signal.
Detecting multiple targets
Multiple labeling is used for the detection of more than one analyte in the same assay. Primary antibodies derived from different species, or mouse monoclonal antibodies of different subclasses, are optimal choices for multiple labeling. If these choices are unavailable, multiple labeling can be achieved through special strategies.
Read more about multiple labeling
Antibody optimization
Primary antibodies may detect the target protein satisfactorily for one experimental protocol, but not work as well under different conditions. It is good practice to optimize and therefore validate the antibody each time the experimental conditions are altered, for example, when the tissue type is changed or a different secondary antibody detection system is used.
Selecting the secondary antibody for IHC and IF
When selecting secondary antibodies for IHC and multiple labeling experiments, consider the criteria in the following table.
The affinity-purified antibodies marked “ML” (multiple labeling) have been specifically prepared to meet these criteria.
Caution: Avoid the possible formation of immune complexes by diluting antibodies in PBS/Tween only (without carrier proteins such as normal serum). To limit unwanted interactions incubate reagents sequentially rather than mixing multiple antibodies.
| Requirement | Antibody | Example |
| The secondary antibody must not recognize endogenous tissue immunoglobulins. | Choose a secondary antibody cross-adsorbed against the species of interest. | In Rat tissue, choose Goat Anti-Mouse IgG (H+L)(min X Hu, Bov, Hrs, Rb, Rat Sr Prot) |
| For visualizing a primary antibody on tissue from the same species (e.g. mouse on mouse labeling) block endogenous Ig with Fab fragments. | See Fab fragment blocking protocols. | |
| The secondary antibody must not bind to endogenous Fc receptors and proteins. | Block with normal serum from the same species as the secondary antibody. | See normal serums. |
| Multiple secondary antibodies must not recognize one another | Use secondary antibodies that are derived from the same host species if possible. | Donkey Anti-Mouse |
| Each secondary antibody must only recognize its target primary antibody. |
For multiple labeling protocols, choose secondary antibodies which have been cross-adsorbed against the other species employed in the experiment. |
Antibodies raised in donkeys have been highly cross-adsorbed against other species. |
| For labeling different mouse subclasses, use subclass specific antibodies. | Goat Anti-Mouse IgG1, IgG2a, etc. See anti-Mouse IgG subclass specific antibodies. | |
| If appropriate cross-adsorbed antibodies are unavailable use a Fab protocol for multiple labeling. | See Fab fragment blocking protocols. |
Detection
Analytes can be visualized under a fluorescence microscope by using a fluorescent conjugate, or under a light microscope by using an enzyme conjugate followed by a chromogenic substrate (colorimetric detection). Detection by light microscopy is also possible using silver enhancement of a colloidal gold antibody.
Each detection method can offer advantages, in terms of sensitivity, quantification, cost-per-assay or capacity for detection of multiple analytes. Table 1 below compares the two detection methods.
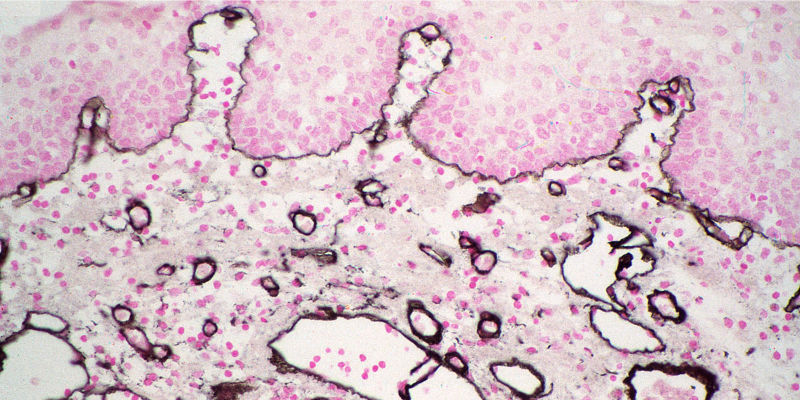
Colorimetric detection
Alkaline Phosphatase (AP) and Horseradish Peroxidase (HRP) conjugates can be used for colorimetric detection. Colorimetric detection can offer quick and easily obtained results, with excellent detection sensitivity. Multiple analyte detection can be achieved by using different chromogens in sequential incubations.
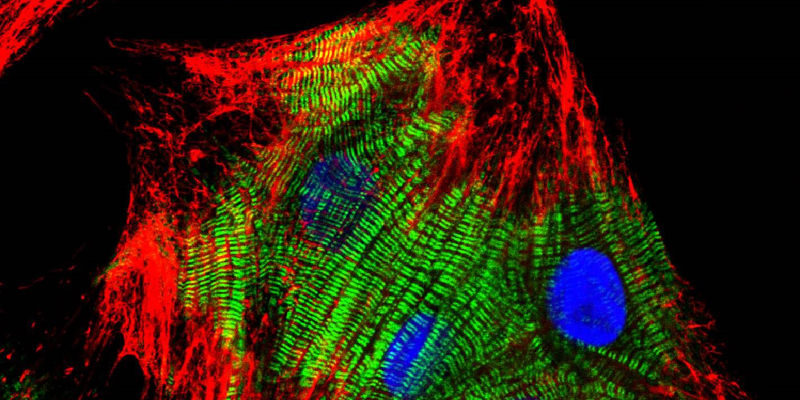
Primary culture of neonatal rat cardiomyocytes stained with Cy™3-AffiniPure Goat Anti-Mouse IgG (H+L) (115-165-146) and Cy™2-AffiniPure Goat Anti-Rabbit IgG (H+L) (111-225-144) secondary antibodies together with DAPI counterstain. Image courtesy of Elisabeth Ehler, KCL.
Fluorescent detection
Immunofluorescence uses a fluorescent dye conjugate to visualize antigens. Fluorescent IHC allows for detection of multiple analytes, and up to 5 color immunofluorescence is possible using the appropriate fluorescent dyes and filter sets. Stripping and reincubating with additional antibodies has resulted in 20 analytes being probed on a single tissue sample.
| Colorimetric | Fluorescent | |
| Sensitivity | HRP conjugates are more sensitive than fluorescent conjugates. | Fluorophores such as Alexa Fluors® and Cyanine dyes are brighter and more photostable than FITC, increasing their sensitivity. |
| ImmunoGold with silver enhancement is also very sensitive. | ||
| Expense and ease | Simple light microscopes are commonly available. | Epifluorescent microscopes are readily available and versatile. Expensive multichannel microscopes may require use of core facilities, increasing cost and difficulty of experiment. |
| Resolution | Detail and depth of interrogation are limited by the quality and thickness of the samples. | Maximum detail can be detected using confocal microscopy, including compilation of 3D images using scanning laser techniques to visualize within much thicker sample slices. |
| Electron microscopy using ImmunoGold reagents offers excellent resolution. | Super-resolution microscopy allows images to be resolved down to 50 nm. | |
| Multiplexing (simultaneous detection of multiple antigens) | Multiple analytes can be labeled by using different chromogens for detection in sequential incubations. | Up to 5 color immunofluorescence can be performed with appropriate microscope laser and filter set up. Additional multiplexing has been reported using rounds of stripping and reincubating. |
| Colocalization | Analytes can be identified by channel, and their signals merged to identify overlap by color. | |
| Color stability | Stains are stable and do not fade during examination under the microscope. Slides can be stored for years. | Photostability can be variable across fluorescent conjugates, making data collection time sensitive. Permanent mounting media can increase longevity of archived slides. |
| Advanced techniques | Fluorescent dyes can be used for a number of techniques to characterize biological interactions. FRET (Förster Resonance Energy Transfer) can be used to examine structural characteristics, binding stoichiometries, and affinities. Super-resolution microscopy allows images to be resolved down to 50 nm.
Fluorescent reagents make it possible to observe live cells in real time. |
|
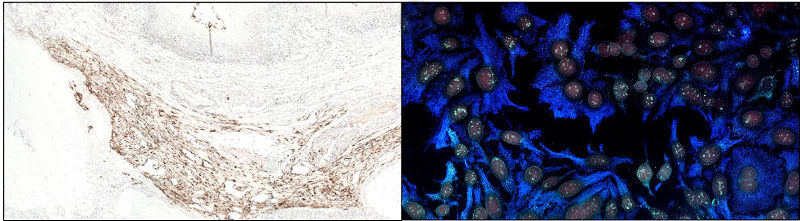
IHC can be performed using colorimetric and fluorescent detection. A: Colorimetric detection using DAB. B: Double immunofluorescence in HEP2 cells. Staining using Brilliant Violet™ 421 and 480, nuclear staining with DRAQ5™.
Blocking reagents
Blocking steps can dramatically improve results and simplify analysis of IHC experiments. To avoid unexpected signal from non-specific, conserved sequence and/or Fc-receptor binding, we recommend blocking with normal serum from the host species of the labeled antibody (5% v/v). Other blocking reagents, such as BSA or commercially available blockers, may also be effective at minimizing background.
Controls
The addition of experimental controls will improve analysis of results and aid troubleshooting.
Read more about blocking and controls
Cell and Tissue Staining with JIR Secondary Antibodies
Suggested dilution ranges for Histo/Cytochemistry are shown in the table below.
| Product | Conjugate | Histo-/Cyto chemistry |
| Whole IgG and F(ab’)2 secondary antibodies | Unconjugated | 10-20 µg/ml |
| Fab secondary antibodies | Unconjugated | 20-40 µg/ml |
| Whole IgG, F(ab’)2 and Fab fragment secondary antibodies | Alexa Fluor® 488, 594, 647, DyLight™ 405, and Cy™3 | 1:100-1:800 |
| Whole IgG, F(ab’)2 and Fab fragment secondary antibodies | AMCA, BV421™, BV480™, Cy2, FITC, TRITC, RRX, and Texas Red | 1:50-1:200 |
| Whole IgG secondary antibodies | Cy™5 | 1:100-1:400 |
| Whole IgG and F(ab’)2 secondary antibodies | Horseradish Peroxidase | 1:500-1:5,000 |
| Whole IgG, F(ab’)2 and Fab secondary antibodies | Biotin-SP (using Fluorophore-Conjugated Streptavidin) | 1:200-1:1,000 |
| Whole IgG and F(ab’)2 secondary antibodies | Biotin-SP (using enzyme-Conjugated Streptavidin) | 1:500-1:5,000 |
| Streptavidin | Horseradish Peroxidase | 1-2 µg/ml |
| Streptavidin | Alkaline Phosphatase | 1-2 µg/ml |
| Streptavidin | Alexa Fluors®, DyLight 405, and Cy3 | 0.5-2 µg/ml |
| Streptavidin | Cy5 | 0.5-2 µg/ml |
| Streptavidin | All Other Fluorophores | 2-5 µg/ml |
| 4 nm Gold-Antibody Complexes | 1:20-1:200 | |
| 6, 12 nm Gold-Antibody Complexes | 1:20-1:40 | |
| 18 nm Gold-Antibody Complexes | 1:10-1:20 | |
| Peroxidase-Anti-Peroxidase (PAP) | 25-50 µg/ml | |
| FabuLight™ Anti-IgM | All Fluorophores | To complex with primary antibody in solution, use 1:1 weight ratio of Fab: primary antibody (15:1 molar ratio). |
| FabuLight Anti-IgG | All Fluorophores | To complex with primary antibody in solution, use 1:1 weight ratio of Fab: primary antibody (3:1 molar ratio). |
| Normal Serum | 5% (v/v) for blocking | |
| ChromPure™ | 10 µg/ml |
Applications of Secondary Antibodies – Flow Cytometry
Flow cytometry is a powerful technique for measuring and analyzing the physical characteristics of single particles in solution as they travel past a beam of light. Properties such as relative size and fluorescence can be measured, and fluorescently tagged antibodies enable cells to be interrogated for multiple proteins and molecular dynamics. Isotype controls are used for experiment validation and analysis of results.
Indirect Flow Cytometry
Flow cytometry can be performed directly, using conjugated primary antibodies, or indirectly, using a conjugated secondary antibody to bind an unconjugated primary. Indirect flow cytometry allows the choice of a wide range of probe molecules, enabling the user to match the desired probe with any primary antibody. Secondary antibody conjugates can improve a flow cytometry experiment by preserving the active site of the primary antibody, and by signal amplification (see Figure 1 B).
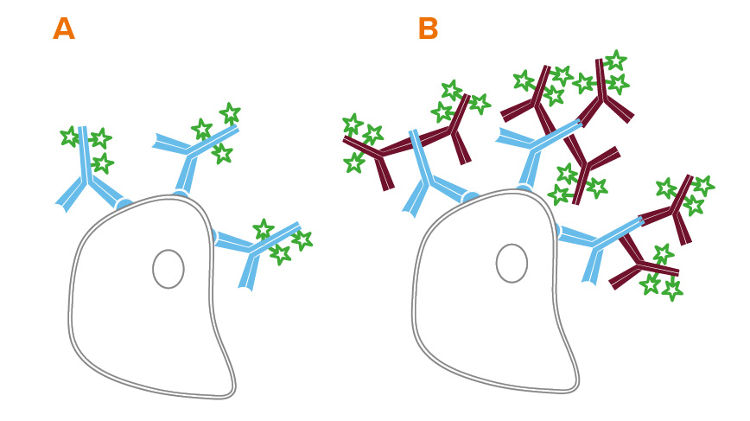
Secondary Antibody Format for Flow Cytometry
The format of secondary antibody can impact the success of an experiment. In addition to whole molecule IgG, Jackson ImmunoResearch offers fragments of secondary antibodies.
F(ab’)2 Fragments
F(ab’)2 fragments are generated by proteolysis of the whole IgG to yield a divalent fragment containing two Fab arms and no Fc domain. When used to stain tissue or cells, the F(ab’)2 secondary antibodies can help to avoid background caused by off-target binding. The absence of an Fc region prevents F(ab’)2 antibodies from being captured by Fc receptors expressed on cell surfaces. Please note that if a primary antibody is trapped by an Fc receptor, the F(ab’)2 secondary antibody will detect the off-target binding, so blocking is critical.
FabuLight™ – Fc Specific Fab Fragments
FabuLight™ secondary antibodies are created by papain digestion of IgG, followed by removal of Fc fragments. These monovalent Fab fragments are specific for the Fc region of primary antibodies, so they don’t interact with the primary’s antigen-binding region. Conjugated FabuLights are convenient for labeling primary antibodies prior to incubation with an experimental sample, saving incubation and wash steps. Like F(ab’)2 fragments, these Fab fragments can minimize background staining due to Fc receptor binding.
Fluorescent Conjugates for Flow Cytometry
The choice of fluorescent dye conjugate depends on a number of experimental variables.
- Instrument capabilities. Consider excitation capabilities (excitation wavelengths or laser colors), and which filters are available.
- Experimental sample. It may be necessary to consider autofluorescence or the expression of recombinant fluorescently tagged proteins, which may preclude using fluorophores with spectral overlap.
- Sensitivity required. Several fluorophores may have similar excitation and emission spectra, but differences in inherent brightness can result in one fluorophore showing a larger population shift. For example, Alexa Fluor® 488 is brighter than FITC.
- Degree of color separation required. For multiple labeling, the dye panel choices will be constrained by the equipment available. To achieve good color separation it is important to look at the emission overlap of the fluorophores in the dye panel. Panel developing tools are available online which can help build dye panels specific to any instrument.
Flow cytometry with JIR Secondary Antibodies
Fluorescent dyes from UV to far red can be used for flow cytometry, depending on instrument capabilities. Jackson ImmunoResearch offers a range of fluorescent dye conjugates spanning the spectrum, making it possible to design a flow cytometry dye panel that accommodates instrument capabilities and recombinant proteins incorporated in the experiment. These secondary antibody conjugates can be found listed in the tables of Whole IgG and F(ab’)2 Fragments.
Fluorescent Protein Conjugates for Flow Cytometry
Jackson ImmunoResearch offers three large, bright fluorescent proteins (R-PE, APC, and PerCP) conjugated to a selection of highly adsorbed secondary antibodies, streptavidin, and purified immunoglobulin controls. The conjugates are excellent choices for surface labeling, but their size may preclude their use as intracellular probes.

Controls for Flow Cytometry
Controls are essential to validate an experiment and interpret results. ChromPure™ proteins are purified from the serum of non-immunized animals and are appropriate experimental controls. An isotype control is a negative control which estimates the non-specific binding of an antibody. Isotype controls are antibodies which match the host species and class of antibodies used in the experiment but are not directed against the antigen of interest. An isotype control should be conjugated with the same reporter molecule as the specific antibody.
Isotype Controls for Flow Cytometry
An isotype control for direct immunofluorescence will be conjugated to the same fluorophore as the primary antibody. For polyclonal primary antibodies (e.g. rabbit or goat primaries), conjugated ChromPure purified proteins are good experimental controls. Conjugated ChromPure proteins can also be used as controls for monoclonal primaries, though it may be preferable to use a control that is the same subclass as the monoclonal.
Indirect immunofluorescence may require isotype controls for both the primary and secondary antibodies
- Unconjugated ChromPure purified proteins can be used as experimental controls for unlabeled polyclonal primary antibodies. Some users choose a control that is the same subclass as their monoclonal primary antibody, but the mixed subclass ChromPure proteins may also be acceptable controls.
- ChromPure purified proteins conjugated with the same reporter molecule as the labeled secondary antibody are good isotype controls. The isotype control should have the same format (whole molecule or antibody fragment) as the secondary antibody.
Suggested Dilution Ranges for Flow Cytometry Applications
The dilutions suggested in the following table are presented as ranges because the optimal dilution is a function of many factors, such as antigen density, permeability, etc. The optimal working dilution should be determined empirically for each application.
| Product | Dilution |
| Unconjugated whole IgG and F(ab’)2 secondary antibodies | 10 - 20 µg / ml |
| Unconjugated Fab secondary antibodies | 20 - 40 µg / ml |
| All Alexa Fluor®, DyLight, and Cy3 Conjugates [IgG, F(ab’)2, Fab] | 1:100 - 1:800 |
| Brilliant Violet™ Conjugates [IgG] | 1:50 - 1:200 |
| AMCA, Cy2, FITC, TRITC, RRX, and Texas Red Conjugates [IgG, F(ab’)2, Fab] | 1:50 - 1:200 |
| Cy5 Conjugates [IgG] | 1:100 - 1:400 |
| Phycoerythrin and Allophycocyanin Conjugates [IgG, F(ab’)2, Streptavidin] | 1:50 - 1:200 |
| PerCP Conjugates [IgG, F(ab’2, Streptavidin] | 1:25 - 1:100 |
| Biotin-SP Conjugates [IgG, F(ab’)2, Fab] | 1:200 - 1:1,000 |
| All Alexa Fluor®-, DyLight 405-, and Cy3-Conjugated Streptavidin | 0.5 - 2 µg / ml |
| Cy5-Conjugated Streptavidin | 1 - 4 µg / ml |
| All Other Fluorophore-Conjugated Streptavidin | 2 - 5 µg / ml |
SRM with JIR Secondary Antibodies
Super-resolution microscopy (SRM) encompasses any optical technique which circumvents the resolution limits of light diffraction found in conventional light microscopy. SRM techniques allow cellular structures to be resolved to the sub-organelle level, enabling information about the 3D structure of cellular components to be determined, and single molecule colocalization to be observed.
Each SRM technique has its own requirements for probe selection. Jackson ImmunoResearch offers a wide selection of labeled secondary antibodies with dyes known to be robust in SRM methods. It should be noted that the field of SRM changes rapidly and guidance for each of the techniques is beyond the scope of this guidance. It is therefore recommended that information from specialized technical literature is sought to aid fluorophore selection.
The following sections briefly outline the principles of some of the most popular SRM techniques.
Stimulated Emission Depletion (STED) microscopy
Diffraction of light by a microscope lens causes light from a single point to appear over a larger area, known as a point spread function or PSF (see Figure 1).
Stimulated emission depletion (STED) microscopy produces super-resolution images by confining the fluorescing region (PSF) of a sample. STED microscopes use two overlapping lasers, the first of which excites the fluorophores as per conventional microscopy (Figure 2A). The second laser, called a depletion laser (STED laser), excites a “donut” of light, with a very small (~30 nm) zero intensity (unexcited) point at its center. This second laser essentially “switches off” the fluorescence generated by the first laser except at the center of the donut, thereby reducing the excited fluorescent molecules to those at the zero point. This effectively reduces the PSF to produce a very small focused region of single molecule fluorescence. Without overlapping interference patterns, high-resolution images can be obtained (Figure 2D). Images with a resolution up to 30 nm in the axial (x-y) plane have been reported.
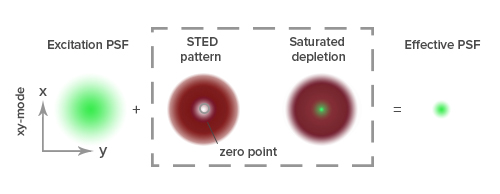
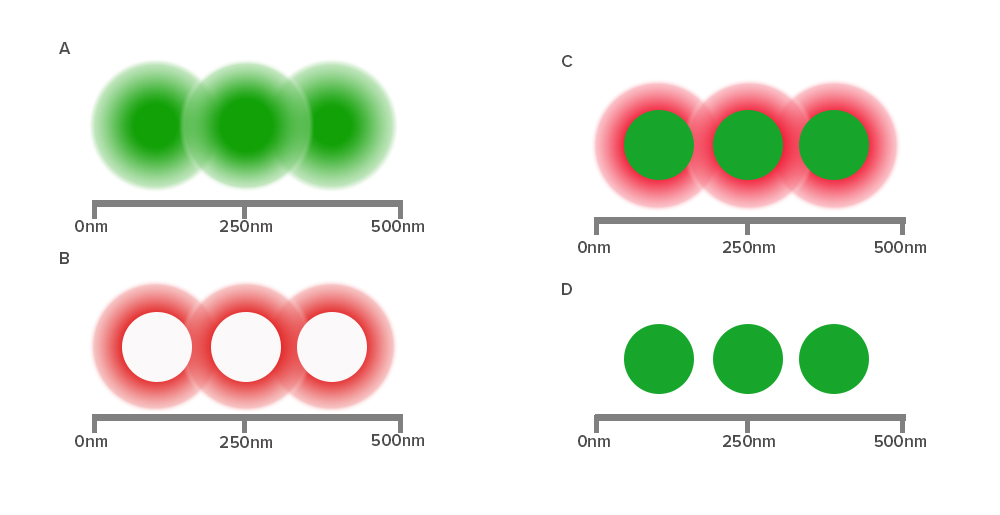
Figure 2:
- Individual proteins closer than 250 nm cannot be resolved by standard confocal imaging, resulting in a blurred image.
- The STED depletion laser creates a “donut” of quenched fluorescence.
- The saturated depletion functionally reduces the excitation PSF.
- The individual proteins are now resolved.
Fluorophore-conjugated antibodies for STED
To achieve super-resolution, dyes must have a high emission cross section with the STED laser wavelength and efficiently achieve a high saturation. This intense illumination ensures that all molecules to be “turned off” by the STED laser are dominated by stimulated emission. Suitable dyes should have a low propensity for photo-bleaching, with high quantum yields and contrast, and contain sufficient density of labeling in close proximity to the target.
Jackson ImmunoResearch offers secondary antibodies conjugated to dyes over a broad spectral range that have been successfully employed in STED: Alexa Fluor® 488, FITC, Alexa Fluor® 594 and Alexa Fluor® 647.
Stochastic Optical Reconstruction Microscopy (STORM)
Single molecule localization can be achieved using Stochastic Optical Reconstruction Microscopy (STORM) using photoswitchable fluorophores to generate images with resolution superior to those collected using conventional methods. There are a number of SRM techniques which exploit the principle of reversible saturable optically linear fluorescence (RESOLFT), using photoswitching or photoactivation of fluorescent dyes. Examples include direct stochastic optical reconstruction microscopy (dSTORM), photoactivated localization microscopy (PALM), fluorescence photoactivation localization microscopy (fPALM), ground state depletion followed by individual molecule return (GSDIM), and super-resolution optical fluctuation imaging (SOFI).
These techniques can be performed using either a pair of dyes to function as an effector and an activator dye to achieve photoswitching, or using dyes which “self-switch” in the case of direct STORM (dSTORM) (Heilemann et al., 2008). Jackson ImmunoResearch provides secondary antibody conjugates suitable for dSTORM applications. Briefly, a relatively low-intensity excitation laser excites the sample, randomly activating a small number of dye molecules. The individually fluorescing dye molecules are spread apart enough so the center of their point spread function can be calculated, which infers the exact location of the dye. A second laser is then used to switch all the molecules off and the process is repeated. High resolution images are then mathematically generated by overlapping all of the mapped point spread functions of each dye.
Fluorophore-conjugated antibodies for single molecule localization experiments
The best dyes for single molecule localization are typically very bright and result in enough photons to reliably produce tight Gaussian distributions. Jackson ImmunoResearch offers several proven dyes in a broad spectral range such as Alexa Fluor® 488, Alexa Fluor® 647 and Cy™5 for use in these types of experiments.
Suggested fluorescent dye conjugates for super-resolution microscopy
| STED | Excitation (nm) | Emission (nm) |
| Alexa Fluor® 488 | 493 | 519 |
| Fluorescein / FITC | 492 | 520 |
| Alexa Fluor® 594 | 591 | 614 |
| Alexa Fluor® 647 | 651 | 667 |
| dSTORM | Excitation (nm) | Emission (nm) |
| Alexa Fluor® 488 | 493 | 519 |
| Alexa Fluor® 647 | 651 | 667 |
| Cy™5 | 650 | 670 |
References
- Huang, B., Bates, M., & Zhuang, X. (2009). Super-resolution fluorescence microscopy. Annual Review of Biochemistry, 78 , 993–1016.
- Farahani, J.N. et al. (2010). Stimulated Emission Depletion (STED) Microscopy: from Theory to Practice. Microscopy: Science, Technology, Applications and Education. 1539-1547.
- Heilemann M, et al. (2008) Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew Chem Int Ed Engl. 47:6172–6176.
- Dempsey, et al. (2011). Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nature Methods, 8, 1027-1036.
ELISA with JIR Secondary Antibodies
An enzyme-linked immunosorbent assay (ELISA) is a robust and sensitive technique used to detect and quantify specific proteins in samples that may contain complex mixtures of proteins. Antibodies are used to detect the specific proteins immobilized on the surface of microplate wells. The technique facilitates high volume and fast throughput analysis, ideal for analyzing large numbers of samples.
ELISA Formats
ELISAs can be performed in a number of ways depending on the sample specifics and the sensitivity required.
Direct ELISA
The sample is applied to a protein-binding solid surface, typically a microtiter plate, coating the analyte directly onto the surface, if present (Fig. A). Next, reporter-conjugated primary antibodies specific to the analyte are added. Signal from the directly conjugated reporter molecule is analyzed to provide a quantitative result when used with a standard curve of known concentration. Depending on the sample characteristics this method may have limited sensitivity.
Indirect ELISA
The analyte is coated directly onto a microtiter plate (Fig. B). The sample (biological fluid: serum, saliva, etc.) is added and antigen-specific antibodies, if present, bind to the analyte, forming a complex. Subsequent washing removes non-binding proteins and other components in the sample mixture. A reporter molecule-conjugated secondary antibody is used to detect the bound antigen-specific antibody. Signal generated may be quantified by comparing to a standard curve.
Sandwich ELISA
Sandwich ELISAs can be performed both directly or indirectly depending on the level of sensitivity required. There are a variety of methods that use the specificity of antibodies to target different fragments of the antibody allowing greater specificity and versatility.
Direct Sandwich
A primary antibody specific to the antigen (analyte) of interest is immobilized onto a microtiter plate and subsequently captures the analyte from the test sample. A reporter-molecule conjugated primary antibody specific to the antigen is added to complete the sandwich. The signal from the reporter molecule is observed either by adding an enzyme substrate, which results in a colorimetric product or fluorescence, resulting in a readout proportional to the analyte concentration.
Indirect Sandwich
A primary antibody specific to the antigen (analyte) of interest is immobilized onto a microtiter plate and subsequently captures the analyte from the test sample (Fig. C). A second primary antibody of a different host species-specific to the antigen is then added to complete the sandwich. A reporter molecule-conjugated secondary antibody binds to the second antigen-specific antibody amplifying signal. The signal from the reporter molecule is observed either by adding an enzyme substrate, which results in a colorimetric product or fluorescence, resulting in a readout proportional to the analyte concentration.
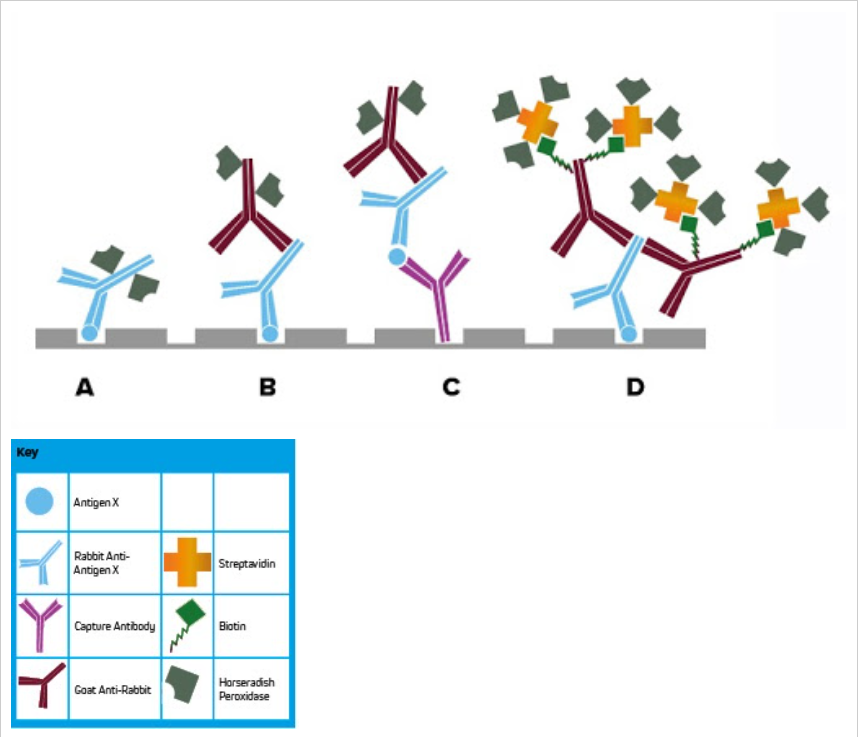
Figure 1:
- Direct ELISA. A conjugated primary antibody detects plate-bound analyte.
- Indirect ELISA. Multiple conjugated secondary antibodies are able to bind the primary antibody, leading to signal amplification.
- Indirect sandwich ELISA. A capture antibody bound to the plate, which binds analyte from the sample, which is then visualized using a conjugated secondary antibody.
- Labeled Streptavidin-Biotin (LSAB) signal amplification. Biotinylated secondary antibodies bind the primary antibody which has reacted with plate-bound analyte. Conjugated streptavidin then binds to multiple biotin molecules on the secondary antibody, leading to maximal signal amplification.
Lateral Flow
Point of care lateral flow tests for the diagnosis of disease utilize the principles of ELISA.
Comparison of ELISA Methods by Step
| Step | Direct | Indirect | Indirect Sandwich | Signal Amplification |
| Coat | Analyte | Capture antibody | ||
| Block | We recommend using 5% normal serum of the species of the detection antibody | |||
| Wash | To remove unbound reagents | |||
| Sample | Conjugated primary antibody | Unconjugated primary | Sample | Sample |
| Wash | To remove unbound reagents | |||
| 2nd | N/A | AP/HRP or fluorophore-conjugated secondary antibody | Biotinylated secondary antibody | |
| Wash | N/A | To remove unbound reagents | ||
| 3rd | N/A | Streptavidin | ||
| Wash | N/A | To remove unbound reagents | ||
| Signal Detection | Colorimetric, chemiluminescent or fluorescent | |||
ELISA for Diagnostics
ELISAs or Enzyme immunoassays (EIA) are frequently used in clinical diagnostic testing; many are validated and available commercially as kits containing all the necessary reagents to perform the test, allowing labs to have access to standardized procedures.
ELISA tests detect immunoglobulins produced as part of an immune or allergic response allowing the diagnosis of infections and allergic diseases, such as food allergy. Alternatively, ELISA can be used to identify causative agents through the detection of the antigen, such as allergens, virus particles, or bacteria, allowing identification of infectious disease.
Typically performed using a polystyrene microtiter plate, the analyte may be coated on the plate, or coated with a capture antibody in the case of a sandwich ELISA. A blocking step using an appropriate serum such as Bovine Serum Albumin (BSA) minimizes the potential for background signal from non-specific interactions between the patient sample and the plate. The patient sample, which may be blood, saliva, or another biological fluid, is introduced to the plate allowing either immunoglobulins or antigens to complex with the capture material. Depending on the format of the assay, signal may be confirmed and quantified by, a reporter molecule-conjugated-primary or secondary antibody, or a biotinylated antigen-specific antibody followed by labeled streptavidin to amplify signal.
Blocking
Blocking reagents are especially important in ELISA. We recommend using 5% (v/v) normal serum derived from the host species of the labeled antibody to block all unsaturated binding sites on the microplate, although BSA may also be appropriate.
Secondary Antibody Conjugates for ELISA
Jackson ImmunoResearch alkaline phosphatase (AP) and horseradish peroxidase (HRP) conjugates can be used for colorimetric assays using a chromogenic substrate. For chemiluminescent detection, a luminol based substrate is commonly used with peroxidase conjugates for highly sensitive detection.
Read More About Reporter Enzyme Conjugates
ELISAs can also be performed using fluorescent conjugates to allow simultaneous detection of multiple primary antibodies derived from different species. By using labeled secondary antibodies each antigen can be distinguished specifically by the individual fluorescent signal. The detection limit for fluorescent ELISA is typically lower than colorimetric or chemiluminescent detection using a reporter enzyme.
Read More About Fluorophore Selection
Labeled Streptavidin with Biotinylated Antibodies for Enhanced Sensitivity
Signal enhancement can be achieved using labeled streptavidin to detect a biotinylated antibody (primary or secondary antibody) (Fig. D). Each antibody can present multiple biotin molecules, which are then able to bind to multiple streptavidin molecules. These combined factors mean that multiple probe molecules are available to either catalyze the detection substrate to its end product or generate fluorescent emission, achieving a brighter signal and greater sensitivity.
ELISA with JIR Secondary Antibodies
We recommend determining appropriate dilution ranges for your experiment, the ranges suggested in the table below are only a guide. We find that higher dilutions may be more appropriate particularly when using the HRP conjugates.
| Product | Conjugate | ELISA |
| Whole IgG and F(ab’)2 secondary antibodies | Unconjugated | 10-20 µg / ml |
| Whole IgG, F(ab’)2 and Fab secondary antibodies | Alexa Fluor® 488, 594, 647, DyLight™ 405, and Cy™3 | 1:100 - 1:800 |
| Whole IgG, F(ab’)2 and Fab secondary antibodies | AMCA, BV421™, BV480™, Cy2, FITC, TRITC, RRX, and Texas Red | 1:50 - 1:200 |
| Whole IgG secondary antibodies | Cy™5 | 1:100 - 1:400 |
| Whole IgG and F(ab’)2 secondary antibodies | Horseradish Peroxidase | 1:5,000 - 1:100,000 |
| Whole IgG and F(ab’)2 secondary antibodies | Alkaline Phosphatase | 1:5,000 - 1:50,000 |
| Whole IgG and F(ab’)2 secondary antibodies | Biotin-SP (using enzyme-Conjugated Streptavidin) | 1:20,000 - 1:400,000 |
| Streptavidin | Horseradish Peroxidase | 1-2 µg / ml |
| Streptavidin | Alkaline Phosphatase | 1-2 µg / ml |
| Normal Serum | 5% (v/v) for blocking | |
| ChromPure™ | 10 µg/ml |
EM with JIR Secondary Antibodies
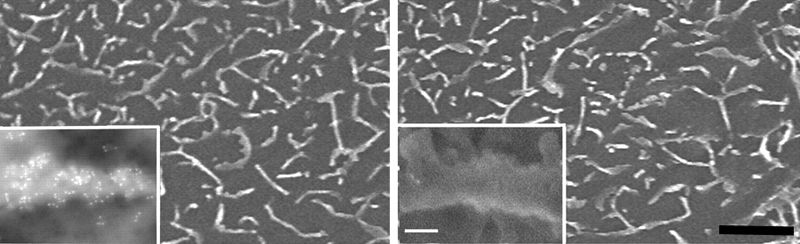
Electron microscopy (EM) allows the collection of high-resolution images using electron beams in the place of light. Staining and immunolabeling with electron dense material enables the visualization of the structures of the sample by adding contrast to the image. Transmission electron microscopy (TEM) requires electrons to pass through thinly sliced specimen sections, allowing 3D images to be compiled and subcellular organelles to be observed. Scanning electron microscopy (SEM) detects the scattered electrons or those emitted from the sample surface. The angle of collection gives the images a 3D quality.
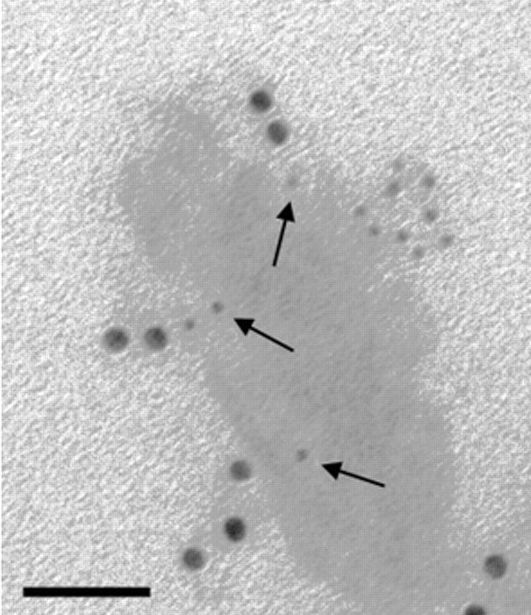
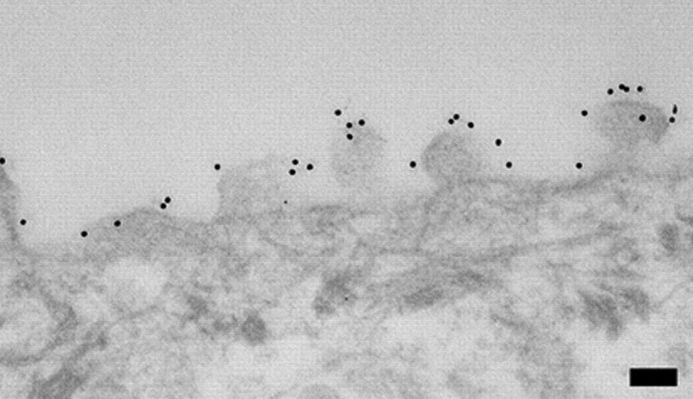
Jackson ImmunoResearch ImmunoGold secondary antibodies for EM
Jackson ImmunoResearch ImmunoGold reagents are colloidal gold particles complexed to secondary antibodies. The electron-dense gold increases electron scatter to reveal high contrast “spots” which allow analytes to be visualized. Labeling of multiple analytes can be achieved by using secondary antibodies complexed to differently sized particles (Figure 1). In addition to TEM and SEM applications, ImmunoGold reagents can be used for brightfield microscopy.
Read more about ImmunoGold reagents for electron microscopy and light microscopy