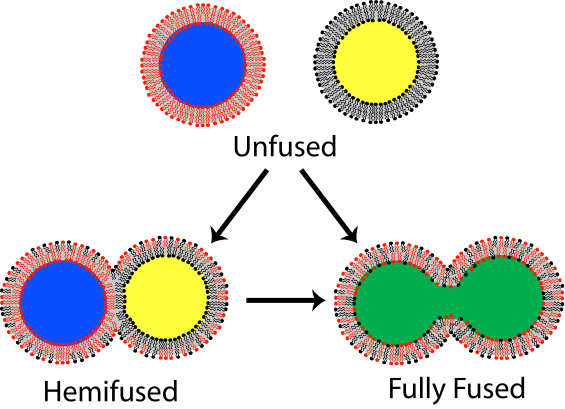
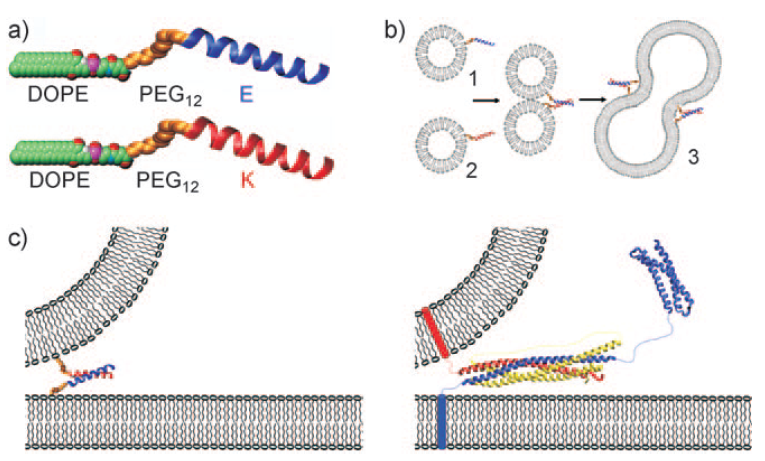
Figure 3: (a) Space-filling model of the two lipidated oligopeptides, LPE and LPK. (b) The two lipidated oligopeptides are spontaneously incorporated into lipid bilayers resulting in liposomes with either LPE or LPK at the surface. When a population of liposomes carrying LPE is mixed with a population of liposomes carrying LPK, the liposomes spontaneously fuse. (c) Diagram showing the fusion of liposomes with the minimal SNARE model of the paper (left) and the SNARE-based protein model (right).
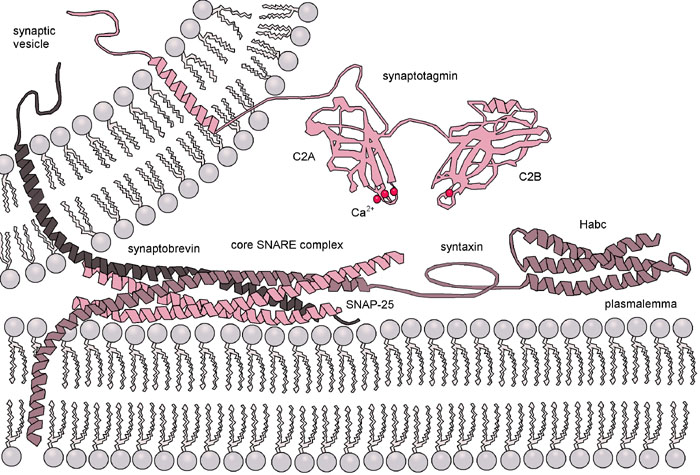
The purpose of this design was to mimic the essential functions of a SNARE protein complex but to minimize the number of different elements needed to direct membrane fusion. The DOPE portion of the construct thus spontaneously anchors the LPE and LPK oligopeptides into the membrane. The dPEG® linker provides a short, flexible spacer that extends the oligopeptides away from the membrane. The two peptides themselves provided the driving force to bring the two membranes together, allowing for membrane fusion.
Using circular dichroism and fluorescence resonance energy transfer (also called Förster resonance energy transfer, known popularly by the acronym FRET) assays with liposome constructs, the researchers were able to distinguish and analyze three distinct stages in membrane fusion. Each stage required an energy input in order to proceed. In the first stage, the liposomes were brought into close proximity. A FRET assay using nitrobenzofuran and lissamine rhodamine dyes attached to the lipid bilayer surfaces showed that the LPE/LPK coiled coil complex that forms spontaneously when the liposomes are in close proximity provides sufficient energy to bring the liposomes together and initiate lipid mixing of the outer lipid layers (known as hemifusion). Further FRET experiments showed that the fusion process initiated by the LPE/LPK coiled coil complex provided enough energy to drive the fusion process to the point of mixing both the outer and inner lipid layers. Furthermore, once the lipid layers were mixed, the contents of the liposomes were observed to mix also.
Conclusions
The approach taken in this paper allowed the research group to build a minimal model of membrane fusion. Indeed, the authors concluded,
“The reduced SNARE model presented herein has been shown to meet all of the characteristics of native membrane fusion, and this similarity combined with the ease of use makes the system a true minimal model for SNARE mediated membrane fusion.” (1) at page 2333
Quanta BioDesign Helps You Build Complex Constructs
This research shows the power of modeling even for complex systems such as membrane fusion. Moreover, this research demonstrates the flexible utility of dPEG® products, in this case PN10283, Fmoc-N-amido-dPEG®12-acid.
If you need to add PEG to your supramolecular construct, why not consider dPEG®? There are numerous advantages to adding PEG to your peptide or protein construct. First, PEG is amphiphilic, providing enhanced solubility in both water and organic solvents. Second, PEG has been proven to reduce aggregation and precipitation of biomolecules. Third, PEG reduces immunogenicity of molecules to which it is conjugated.
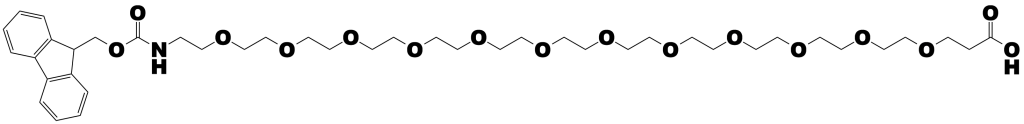
Dispersity is the key advantage of using Quanta BioDesign’s dPEG® products instead conventional PEG products. Conventional PEG is dispersed. Quanta BioDesign’s dPEG® products are single molecular weight compounds. That is, rather than having a single discrete molecular size and weight, a conventional PEG is composed of numerous PEG molecules. The “weight” of a conventional PEG is expressed as an average molecular weight across the range of sizes. Quanta BioDesign’s dPEG® constructs — designed, patented, and manufactured in the United States — are single molecular weight PEG molecules functionalized with various chemical moieties. Whereas conventional PEGs confound analysis by presenting an intractable mixture of different size molecules, dPEG® constructs enhance analysis by being a single, discrete molecule that can be accurately characterized using ordinary analytical chemistry tools.