SNARE-Mediated Membrane Fusion and dPEG®, Part 2
For Part 1 of this three-part series, click here. For part 3, click here.
Introduction
Continuing earlier work, researchers at the laboratory of Alexander Kros examined the parameters that affect membrane fusion in liposomes composed of the minimal model developed in their earlier paper (1). In this paper (2), the research group demonstrated that, using the model system previously developed, “the rate and extent of fusion and the number of fusion rounds can be manipulated by adjusting the fusogen and liposome concentrations” (quote from the abstract).
The SNARE protein model for membrane fusion developed by the research group contained 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine (DOPE) connected to one of two oligopeptides (LPE or LPK) via a dPEG®12 linker derived from PN10283, Fmoc-N-amido-dPEG®12-acid, where the PEG linker served as a means to provide a flexible spacer between the DOPE and the oligopeptides. See Figure 1, below. The coiled-coil forming oligopeptides each contain three-heptad repeats, and when populations of liposomes containing the LPE and LPK constructs are mixed, they associate and lead cleanly to liposome fusion of the two different liposome populations. As discussed in the earlier post on this subject, the model system developed by Kros and colleagues is an accurate, minimalist model of SNARE-mediated liposome fusion.
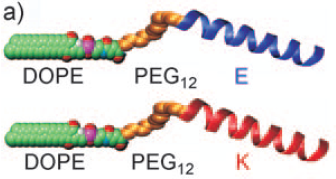
Figure 1: Structure of the DOPE-PEG12-LPE/LPK lipidated oligopeptides used to study liposome fusion.
For this research, the authors characterized the influence on liposome fusion of lipopeptide concentration, lipid concentration, and lipids of positive curvature. The authors also described how liposome fusion in the model system varies with liposome collision, docking, and lipid mixing rates.
How Liposome Fusion Works
The group first studied whether liposome fusion was targeted (that is, molecular recognition between two different fusogens) was required for fusion to occur or non-targeted (no molecular recognition required). This analysis was carried out by mixing populations of liposomes not modified with either LPE or LPK with populations of liposomes carrying either LPE or LPK. In these experiments, no liposome fusion occurred. However, in experiments in which LPE-modified liposomes were mixed with LPK-modified liposomes, liposome fusion occurred. This indicated that molecular recognition between the LPK and LPE peptides was required for liposome fusion to occur; hence, the fusion was targeted.
Next, the researchers examined the effect of concentration of the two fusogens on liposome fusion. By mixing decreasing proportions (mole%) of the lipopeptide fusogens in a fixed amount of total lipids, the scientists were able to determine the lower limit and optimal amount of fusogens required for liposome fusion. By this method, it was determined that 0.05 mole% lipopeptide was the lower limit of fusogens required for at least one round of liposome fusion to occur. The optimal amount of fusion was found to be 0.75 mole% LPE or LPK incorporated into the liposome membranes. Circular dichroism data demonstrated that the lipidated oligopeptides (LPE and LPK) were most likely distributed evenly across the membrane surface at 0.75 mole%.
The general trend of increasing membrane fusion rates with increasing proportions of fusogens arises because two liposomes that diffuse into close proximity are more likely to be displaying complementary peptides in the correct orientation for binding in the approaching area, and hence are more likely to dock and undergo fusion. (2) at page 1048.
Above 0.5 mole% lipopeptide concentration, the researchers found that completely lipid mixing occurred rapidly. Large particles (“giant liposomes”) and clusters of docked and fused particles formed, and the particles sedimented in a matter of hours. Conversely, at 0.25 mole% or less lipopeptide concentration, the particles that form from liposome fusion are initially small, increase in hydrodynamic diameter size, then decrease again over time before plateauing “at the size corresponding to the fusion of two liposomes in which the volume is conserved rather than the outer surface area” (2, p. 1050). This indicates that the SNARE mimetic liposome fusion is “clean,” meaning that no loss of content occurs during fusion.
The research group analyzed whether lipid concentration affected membrane fusion, and if so, by how much. Using FRET experiments, the group found that complete lipid mixing was more efficient if there were more fusogens (the DOPE-PEG12-oligopeptides) on fewer liposomes than if there were fewer fusogens on more liposomes. A lower limit of 0.025 mM lipids (0.25 µM lipopeptide) was established as the lower limit of lipid concentration at which liposome fusion occurred efficiently.
Finally, the group examined the effect of changing lipid curvature on liposome fusion. Using the positive curvature lipid lysophosphatidylcholine (LPC) instead of DOPE altered the shape of the liposomal surface and prevented formation of the so-called “stalk intermediate” that is required in SNARE-mediated fusion (the stalk intermediate requires a negative curvature in order to form). With as little as 15 mole% LPC incorporated into liposomes, the researchers found that lipid mixing decreased dramatically; however, docking (the first step in liposome fusion) was not inhibited, but hemifusion and full fusion were prevented from occurring.
The research performed provided detailed understanding of how the reduced SNARE model works to promote liposome fusion. This detailed degree of understanding is important for developing applications of liposome fusion (or membrane fusion) such as targeted delivery of drugs to cells and controlled mixing of reagents at nanoliter volumes, such as would occur in a lab-on-a-chip application.
dPEG® Role in This Research
As mentioned in an earlier post, the DOPE-PEG12-LPE/LPK constructs used PN10283, Fmoc-N-amido-dPEG®12-acid to create the flexible extension between the lipid membrane surface and the oligopeptides. If you need to add PEG to your supramolecular construct, why not consider dPEG®? There are numerous advantages to adding PEG to your peptide or protein construct. First, PEG is amphiphilic. Second, PEG reduces aggregation and precipitation of biomolecules. Third, PEG diminishes the immunogenicity of molecules to which it is conjugated. Conventional PEG, though, is polydispersed, meanign that it is composed of numerous PEG molecules. The “weight” of a conventional PEG is expressed as an average molecular weight across the range of sizes. Quanta BioDesign’s dPEG® constructs — designed, patented, and manufactured in the United States — are single molecular weight PEG molecules functionalized with various chemical moieties. Whereas conventional PEGs confound analysis by presenting an intractable mixture of different size molecules, dPEG® constructs enhance analysis by being a single, discrete molecule that can be accurately characterized using ordinary analytical chemistry tools.
We encourage you to look at all our products and find what you need for your next research project. You’ll be pleasantly surprised at what you can do with dPEG®!
References
(1) Hana Robson Marsden, Nina A. Elbers, Paul H. H. Bomans, Nico A. J. M. Sommerdijk, and Alexander Kros. A Reduced SNARE Model for Membrane Fusion. Angew. Chem. Int. Ed (2009), 48, 2330-2333. DOI: 10.1002/anie.200804493
(2) Hana Robson Marsden, Alexander V. Korobko, Tingting Zhen, Jens Voskuhl, and Alexander Kros. Controlled liposome fusion mediated by SNARE protein mimics. Biomaterials Science (2013), 1, 1046-1054. DOI: 10.1039/c3bm60040h
