Apoptosis, or programmed cell death, is a key part of embryonic development and tissue homeostasis in multicellular organisms. Cells undergoing apoptosis are characterized by distinct morphological changes and energy-dependent biochemical mechanisms. These characteristics include membrane blebbing, cell shrinkage, activation of caspase cascade, chromatin condensation and DNA fragmentation, as well as the production of membrane-bound apoptotic bodies. Since no single parameter entirely defines apoptosis, many different approaches are used in the study of cell death.
Apoptosis and Necrosis
Alterations in the plasma membrane is a hallmark indicator of early-stage apoptosis in living cells. During the initial phases of apoptosis, phosphatidylserine (PS) residues translocate from the inner to the outer-leaflet of the plasma membrane. In cell imaging and flow cytometry, fluorescently labeled annexin V conjugates are commonly used to detect apoptotic cells by its ability to bind to externalized PS residues.
Annexin V
Annexins are a family of caclium-dependent phospholipid-binding proteins. They are found in abundance in eukaryotic organisms (animal, plant and fungi) where they play critical roles in various cellular and physiological processes such as signal transduction, vesicle transport, and membrane scaffolding.
Annexin V is a 35 kDa phospholipid-binding protein. Its strong calcium-dependent affinity for phosphatidylserine (PS) can be used to identify cells undergoing apoptosis. In viable cells, negatively charged PS residues are located on the cytosolic surface of the plasma membrane. However, during early apoptosis, cells loose plasma membrane asymmetry and PS residues translocate to the outer leaflet of the membrane, where they can be detected by fluorescently labeled annexin V conjugates.
Table 1. Overview of annexin V conjugates.
| Specification | Value |
| Application | Detects externalized phosphatidylserine to identify apoptotic cells |
| Readout | Fluorescence microscopy, live cell imaging and flow cytometery |
| Live Cells | Yes |
| Fixed Cells | Not for use |
| Fixable | Yes, fix after staining with formaldehyde |
Annexin V Conjugates for Detecting Apoptosis
Annexin V conjugated to iFluor™ dyes provide a fast and reliable method for detecting externalized PS, a key characteristic in early-stage apoptosis. The superior brightness and photostability of Annexin V iFluor™ probes significantly outperforms most Alexa Fluor® and other spectrally similar annexin v conjugates. Annexin V iFluor™ conjugates are useful for live cell imaging, immunofluorescence, and flow cytometry, and can be combined with other dyes, such as nucleic acid stains, to accurately assess mixed populations of apoptotic, necortic and dead cells.
AAT Bioquest offers a wide variety of fluorescently labeled annexin V conjugates in colors ranging from UV to infrared for improved multiplexing flexibilty. We also offer recombinant annexin V and easy-to-use kits optimized for flow cytometry, as well as, biotinylated annexin V, which can be detected using fluorescently labeled streptavidins.

Figure 1. Jurkat cells were treated with 1 µM staurosporine for 4 hours to induce apoptosis. Following treatment, cells were stained with Annexin V-iFluor™ 555 conjugate (Cat No. 20072). The nuclei of dead cells were labeled using Nuclear Green™ DCS1 (Cat No. 17550). Images were acquired on a confocal microscope.
Table 2. Available annexin V conjugates for apoptosis detection.
| Annexin V Label | Ex (nm) | Em (nm) | Filter Set | ε¹ | Φ² | Unit Size | Cat No. |
| iFluor™ 350 | 345 | 450 | DAPI | 20000 | 0.95 | 100 tests (200 µL) | 20070 |
| iFluor™ 488 | 491 | 516 | FITC | 75000 | 0.9 | 100 tests (200 µL) | 20071 |
| iFluor™ 555 | 557 | 570 | TRITC | 100000 | 0.64 | 100 tests (200 µL) | 20072 |
| iFluor™ 594 | 588 | 604 | Texas Red | 180000 | 0.53 | 100 tests (200 µL) | 20073 |
| iFluor™ 633 | 640 | 654 | Texas Red | 250000 | 0.29 | 100 tests (200 µL) | 20073 |
| iFluor™ 647 | 656 | 670 | Cy5 | 250000 | 0.25 | 100 tests (200 µL) | 20074 |
| iFluor™ 680 | 684 | 701 | Cy5.5 | 220000 | 0.23 | 100 tests (200 µL) | 20075 |
| iFluor™ 700 | 690 | 713 | Cy5.5 | 220000 | 0.23 | 100 tests (200 µL) | 20077 |
| iFluor™ 750 | 757 | 779 | Cy7 | 275000 | 0.12 | 100 tests (200 µL) | 20076 |
| iFluor™ 750 | 757 | 779 | Cy7 | 275000 | 0.12 | 100 tests (200 µL) | 20076 |
| AF350 (Alexa Fluor® 350 equivalent) | 343 | 441 | DAPI | 19000 | N/D³ | 100 tests (200 µL) | 20090 |
| AF488 (Alexa Fluor® 488 equivalent) | 499 | 520 | FITC | 73000 | 0.92 | 100 tests (200 µL) | 20092 |
| AF594 (Alexa Fluor® 594 equivalent) | 590 | 618 | Texas Red | 92000 | 0.66 | 100 tests (200 µL) | 20096 |
| FITC | 491 | 516 | FITC | 73000 | 0.79 | 100 tests (200 µL) | 20030 |
| TRITC | 544 | 570 | TRITC | 100000 | 0.1 | 100 tests (200 µL) | 20031 |
| Cy3 | 555 | 569 | Cy3/TRITC | 150000 | 0.04 | 100 tests (200 µL) | 20065 |
| Cy5 | 656 | 670 | Cy5 | 250000 | 0.25 | 100 tests (200 µL) | 20066 |
| Cy5.5 | 684 | 701 | Cy5.5 | 220000 | 0.23 | 100 tests (200 µL) | 20067 |
| Cy7 | 756 | 779 | Cy7 | 250000 | 0.3 | 100 tests (200 µL) | 20068 |
- ε = molar extinction coefficient at their maximum absorption wavelength (Units = cm-1M-1).
- Φ = fluorescence quantum yield in aqueous buffer (pH 7.2).
- N/D = not determined.
Annexin V Conjugates for Flow Cytometry
Annexin V conjugated to mFluor™ dyes, PacBlue, APC and PE are valuable tools for measuring apoptosis by flow cytometry. These conjugates can be combined with other dyes, such as nucliec acid stains, to accurately assess mixed populations of apoptotic, necortic and dead cells. We offer annexin V conjugates for flow cytomtery as stand alone reagents or easy-to-use kits.
Key features of Annexin V mFluor™ Conjugates
- Superior brightness and improved photostability for long-term cellular imaging
- pH-insensitive fluorescence over a wide molar range
- Large Stokes shifts to reduce cross-talk between the excitation source and fluorescence emission for cellular imaging with high signal-to-noise ratios
- Compatible with live cells only
- Fixable after staining with formaldehyde
- Conjugates efficiently excited by common lasers for increased multiplexing capabilities

Figure 2. The detection of binding activity of Annexin V-mFluor™ Violet 450 conjugate to phosphatidylserine in Jurkat cells. Jurkat cells were treated without (Green) or with 1 µM staurosporine (Red) in 37°C for 4 hours, and then ed with Annexin V-mFluor™ Violet 450 conjugate for 30 minutes. Fluorescence intensity was measured using ACEA NovoCyte flow cytometer in Pacific Blue channel.
Table 3. Available annexin V conjugates for optimized for flow cytometry.
| Annexin V Label | Laser | Ex (nm) | Em (nm) | Channel | ε¹ | Φ² | Unit Size | Cat No. |
| PacBlue | Violet Laser | 410 | 455 | DAPI/FL-1 Channel | 46000 | 0.78 | 100 tests (200 µL) | 20089 |
| mFluor™ Violet 450 | Violet Laser | 403 | 454 | DAPI/FL-1 Channel | 25000 | 0.92 | 100 tests (200 µL) | 20080 |
| mFluor™ Violet 510 | Violet Laser | 414 | 510 | FITC/FL-1 Channel | 30000 | 0.86 | 100 tests (200 µL) | 20081 |
| mFluor™ Violet 540 | Violet Laser | 424 | 560 | PE/FL-2 Channel | 15000 | 0.64 | 100 tests (200 µL) | 20082 |
| mFluor™ Blue 570 | Blue Laser | 533 | 570 | PE/FL-2 Channel | 120000 | 0.08 | 100 tests (200 µL) | 20085 |
- ε = molar extinction coefficient at their maximum absorption wavelength (Units = cm-1M-1).
- Φ = fluorescence quantum yield in aqueous buffer (pH 7.2).
Cell Meter™ Annexin V Binding Apoptosis Assays
Cell Meter™ Annexin V Binding Apoptosis Assay Kits provide a fast and convenient method for measuring apoptotic cells based on the externalization of phosphatidylserine. Each kit contains a recombinant annexin V conjugated to either an iFluor™ dye, mFluor™ dye, APC, PE or FITC and used to detect translocated PS in cells undergoing early apoptosis. In addition, these kits (excluding 22825, 22826 and 22827) include a ready-to-use solution of propidium iodide (PI) or Nuclear Red™ DCS1 nucleic acid stain. Both PI and Nuclear Red™ DCS1 are impermeant to live cells but stain necrotic and dead cells with intense red fluroescence. After staining cells with annexin V conjugate and nucleic acid stain, apoptotic cells show annexin V conjugate fluorescence, dead cells show red and annexin V conjugate fluorescence, and live cells show little or no fluorescence. Cell populations can readily be differentiated using a flow cytometer.
Key features of Cell Meter™ Annexin V Bindinng Apoptosis Assays
- High Sensitivity – annexin V labeled with iFluor™ or mFluor™ dyes produce significantly brighter and more photostable signals than Alexa Fluor and other spectrally similar annexin V conjugates
- Flexibility – wide range of fluorescently labeled annexin V conjugates to match any instrument set-up
- Convenient design – streamlined assay procedure which can be completed in 30 to 60 minutes
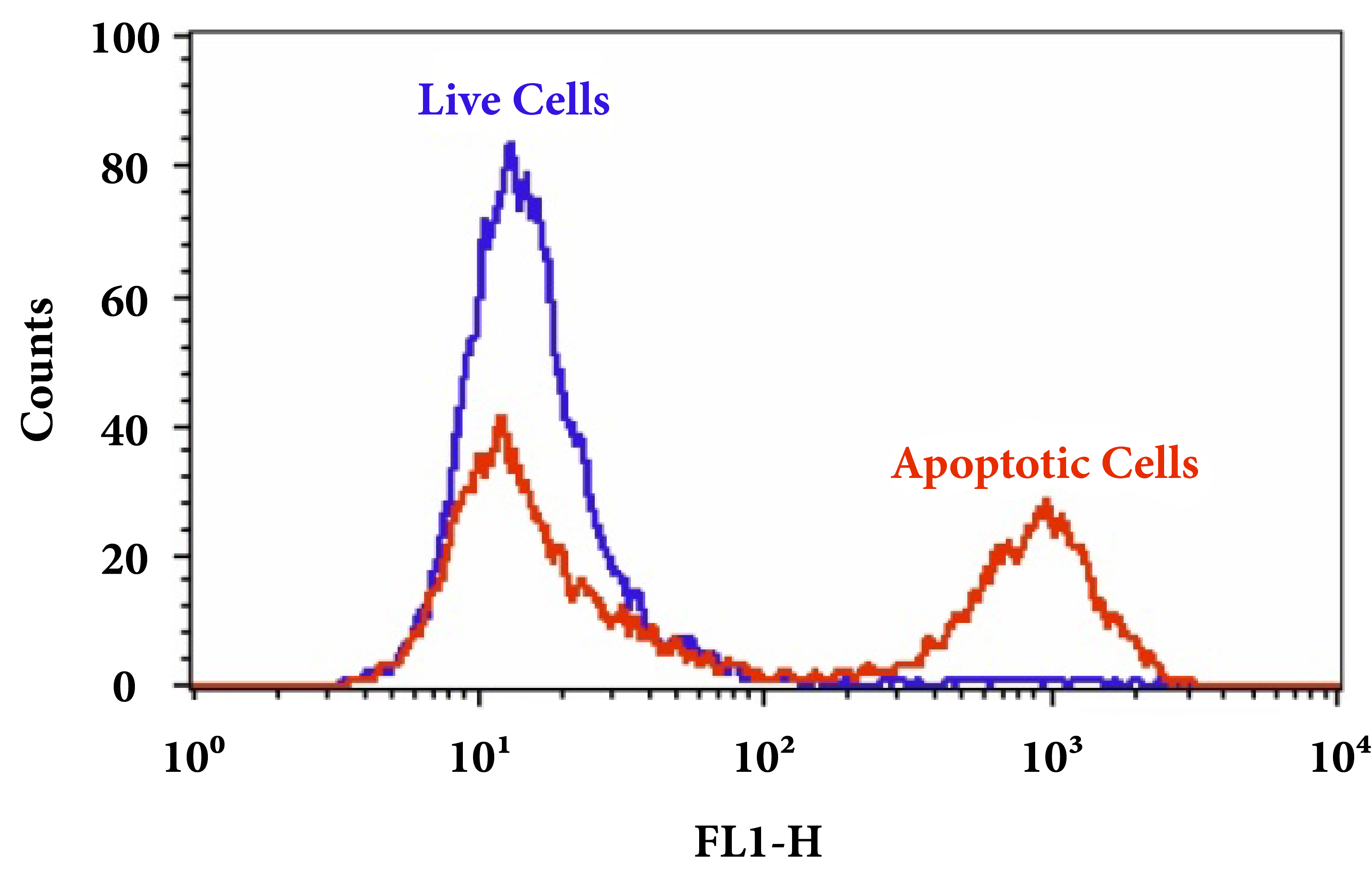
Figure 3. The detection of binding activity of Annexin V-mFluor™ Violet 450 conjugate to phosphatidylserine in Jurkat cells. Jurkat cells were treated without (Green) or with 1 µM staurosporine (Red) in 37°C for 4 hours, and then ed with Annexin V-mFluor™ Violet 450 conjugate for 30 minutes. Fluorescence intensity was measured using ACEA NovoCyte flow cytometer in Pacific Blue channel.
Table 4. Cell Meter™ Annexin V Binding Apoptosis Assay Selection Guide
| Annexin V Conjugate | Dead Cell Stain | Annexin V Conjugate Ex/Em | Dead Cell stain Ex/Em | Unit Size | Cat No. |
| Annexin V-iFluor™ 488 | PI | 490/ 520 nm | 534/617 nm | 100 Tests | 22824 |
| Annexin V-iFluor™ 555 | None | 554/578 nm | *** | 100 Tests | 22825 |
| Annexin V-iFluor™ 594 | None | 590/610 nm | *** | 100 Tests | 22826 |
| Annexin V-iFluor™ 647 | None | 650/668 nm | *** | 100 Tests | 22827 |
| Annexin V-mFluor™ Violet 450 | PI | 405/450 nm | 534/617 nm | 100 Tests | 22828 |
| Annexin V-mFluor™ Violet 500 | PI | 414/508 nm | 534/617 nm | 100 Tests | 22829 |
| Annexin V-mFluor™ Violet 550 | PI | 424/560 nm | 534/617 nm | 100 Tests | 22830 |
| Annexin V-APC | PI | 651/662 nm | 534/617 nm | 100 Tests | 22837 |
| Annexin V-PE | Nuclear Red™ DCS1 | 565/575 nm | 631/651 nm | 100 Tests | 22838 |
| Annexin V-FITC | PI | 490/520 nm | 534/617 nm | 100 Tests | 22839 |
***For Cat No. 22825, 22826 and 22827 use Nuclear Blue™ DCS1 as a dead cell stain.
Product Ordering Information
Ordering information for recombinant annexin V, annexin V conjugates and annexin V assay kits.
| Cat No. | Product Name | Unit Size |
| 20014 | Annexin V, Recombinant | 100 ug |
| 20015 | Annexin V, Recombinant | 1 mg |
| 20018 | Annexin V-Biotin conjugate | 100 tests |
| 20030 | Annexin V, FITC Labeled | 100 tests |
| 20031 | Annexin V, TRITC Labeled | 100 tests |
| 20065 | Annexin V-Cy3 conjugate | 100 tests |
| 20066 | Annexin V-Cy5 conjugate | 100 tests |
| 20067 | Annexin V-Cy5.5 conjugate | 100 tests |
| 20068 | Annexin V-Cy7 conjugate | 100 tests |
| 20069 | Annexin V-iFluor™ 633 conjugate | 100 tests |
| 20070 | Annexin V-iFluor™ 350 conjugate | 100 tests |
| 20071 | Annexin V-iFluor™ 488 conjugate | 100 tests |
| 20072 | Annexin V-iFluor™ 555 conjugate | 100 tests |
| 20073 | Annexin V-iFluor™ 594 conjugate | 100 tests |
| 20074 | Annexin V-iFluor™ 647 conjugate | 100 tests |
| 20075 | Annexin V-iFluor™ 680 conjugate | 100 tests |
| 20076 | Annexin V-iFluor™ 750 conjugate | 100 tests |
| 20077 | Annexin V-iFluor™ 700 conjugate | 100 tests |
| 20080 | Annexin V-mFluor™ Violet 450 conjugate | 100 tests |
| 20081 | Annexin V-mFluor™ Violet 510 conjugate | 100 tests |
| 20082 | Annexin V-mFluor™ Violet 540 conjugate | 100 tests |
| 20085 | Annexin V-mFluor™ Blue 570 conjugate | 100 tests |
| 20089 | Annexin V, PacBlue conjugate | 100 tests |
| 20090 | Annexin V, AF350 conjugate | 100 tests |
| 20092 | Annexin V, AF488 conjugate | 100 tests |
| 20096 | Annexin V, AF594 conjugate | 100 tests |
| 22824 | Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Green Fluorescence Optimized for Flow Cytometry* | 100 tests |
| 22825 | Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Orange Fluorescence Optimized for Flow Cytometry* | 100 tests |
| 22826 | Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Red Fluorescence Optimized for Flow Cytometry* | 100 tests |
| 22827 | Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Deep Red Fluorescence Optimized for Flow Cytometry* | 100 tests |
| 22828 | Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Blue Fluorescence Excited at 405 nm* | 100 tests |
| 22829 | Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Green Fluorescence Excited at 405 nm* | 100 tests |
| 22830 | Cell Meter™ Annexin V Binding Apoptosis Assay Kit *Orange Fluorescence Excited at 405 nm* | 100 tests |
| 22837 | Cell Meter™ APC-Annexin V Binding Apoptosis Assay Kit *Optimized for Flow Cytometry* | 100 tests |
| 22838 | Cell Meter™ PE-Annexin V Binding Apoptosis Assay Kit *Optimized for Flow Cytometry* | 100 tests |
| 22839 | Cell Meter™ FITC-Annexin V Binding Apoptosis Assay Kit *Optimized for Flow Cytometry* | 100 tests |
Probes for Measuring Caspase Activity
Caspases (cysteine-aspartic proteases or cysteine-dependent aspartate directed proteases) are a family of protease enzymes whose functions are intimately linked with the processes of apoptosis (programmed cell death), necrosis, and pyroptosis (inflammation). AAT Bioquest offers a plethora of reagents and kits for measuring caspase activity in real-time enzyme kinetic and end-point assay formats.
What Are Caspases?
Caspases are cytosolic aspartate-specific, cysteine proteases which serve as the primary mediators of apoptosis. A variety of receptors, such as the TNF-? receptor, FasL receptor, TLR and death receptors, as well as, the Bcl-2 and inhibitor of apoptosis (IAP) protein families participate and regulate this caspase-dependent apoptosis pathway. Once activated by an upstream stimulus (extrinsic or intrinsically), caspases execute proteolysis of downstream protein substrates, triggering a ‘cascade’ of events that lead to cell disassembly, death, and the phagocytosis and removal of cellular debris.
Human Caspases
The human caspase family is divided into three main groups, primarily based on commonalities such as sequence similarity and biological function. Group 1 comprises of ‘inflammatory’ caspases with a long caspase-recruitment domain and affinity for large aromatic or hydrophobic residues at the P4 position. Group 2 comprises of ‘apoptotic effector’ caspases with a short pro-domain, while group 3 comprises of ‘apoptotic initiator’ caspases with a long pro-domain and affinity for substrates with leucine or valine at the P4 position (Table 1).
Table 1. Functional classification of human caspases.
| Cell Death Pathway | Type of Caspase | Enzyme | Organism |
| Apoptosis | Initiator | Caspase 2 | Human & Mouse |
| Apoptosis | Initiator | Caspase 8 | Human & Mouse |
| Apoptosis | Initiator | Caspase 9 | Human & Mouse |
| Apoptosis | Initiator | Caspase 10 | Human |
| Apoptosis | Effector | Caspase 3 | Human & Mouse |
| Apoptosis | Effector | Caspase 6 | Human & Mouse |
| Apoptosis | Effector | Caspase 6 | Human & Mouse |
| Pyroptosis | Inflammatory | Caspase 1 | Human & Mouse |
| Pyroptosis | Inflammatory | Caspase 4 | Human |
| Pyroptosis | Inflammatory | Caspase 5 | Human |
| Pyroptosis | Inflammatory | Caspase 11 | Mouse |
| Pyroptosis | Inflammatory | Caspase 12 | Some Humans & Mouse |
| Pyroptosis | Inflammatory | Caspase 13 | Bovine |
Initiator and Effector Caspases
Depending on their role in the apoptosis caspase pathway, caspases can be divided into two categories: initiator and effector caspases. Both initiator and effector caspases bear a catalytic site comprised of a small and a large subunit. The recognition site for caspases is marked by three to four amino acids followed by an aspartic acid residue, with the cleavage occurring after the aspartate. The caspase proteases are typically synthesized as inactive precursors. Inhibitor release or cofactor binding activates the caspases through cleavage at internal aspartates, either by autocatalysis or by the action of another protease. Once activated initiator caspases cleave and activate effector caspases. Activated effector caspases subsequently cleave a number of protein substrates to trigger apoptosis.
Apoptotic initiator caspases such as caspase-2, -8, -9, and -10 can initiate the caspase activation cascade. Caspase-8 is essential for the formation of the death-inducing signaling complex (DISC) and when activated, caspase-8 activates downstream effector caspases (e.g. caspase 3) and mediates the release of cytochrome c from the mitochondria. Caspase-8 has proven to show relatively high substrate selectivity to the IETD peptide sequence. Apoptotic effector caspases such as caspase-3, -6, and -7 may not be responsible for initiating the cascading pathway but when activated, they play an integral role in the intermediate and later steps of the cascade. Caspase-3 (CPP32/apopain) is a key effector as it amplifies the signal from an initiator caspases and signifies full commitment to cellular disassembly. In addition to cleaving other caspases in the enzyme cascade, caspase 3 has been shown to cleave poly(ADP-ribose) polymerase (PARP), DNA-dependent protein kinase C? and actin. Detection of activated caspase-3 is carried out using a DEVD peptide sequence which is selective for caspase-3 and proven successful at developing caspase-3 substrates.
Caspase Substrates & Inhibitors
Caspase substrates and inhibitors consists of two key components: a caspase recognition sequence and either a signal generating or an protease inhibiting motif. The caspase recognition sequence is marked by three or four amino acids that are specific to the caspase or caspases being measured (Table 2). The N-terminus of caspase recognition sequence are typically modified with either acetyl (Ac) or carbobenzoxy (Z) groups to enhance membrane permeability. The intended caspase recognizes the specific peptide sequence as its enzymatic cleavage site, releasing the signal generating or inhibiting motif. Both chromogenic and fluorogenic caspase substrate operate in similar fashion in which signal or color intensity is proportional to the proteolytic activity.
Table 2. Peptide recognition sequences for caspase activity For Caspase Substrates & Inhibitors
| Peptide | Amino Acid Sequence | Target for Caspases |
| IETD | Ile-Glu-Thr-Asp | Caspase 8, Granzyme B |
| DEVD | Asp-Glu-Val-Asp | Caspase 3, 6, 7, 8, or 10 |
| LEHD | Leu-Glu-His-Asp | Caspase 9 |
| VAD | Val-Ala-Asp | Caspase 1, 2, 3, 6, 8, 9 or 10 |
Chromogenic Caspase Substrates
Chromogenic caspase substrates comprise of a signal generating motif, such as the highly colored chromophore pNA (para-nitroaniline or 4-nitroaniline) linked to a caspase recognition sequence. which is monitored colorimetrically at 405 nm using either an absorbance microplate reader or a spectrophotometer.
Fluorogenic Caspase Substrates
The structure of fluorogenic caspase substrates comprises of a fluorophore such as 7-Amino-4-methylcoumarin (AMC), 7-Amino-4-trifluoromethylcoumarin (AFC), Rhodamine 110 (R110) or ProRed™ 620 that is linked to a caspase recognition sequence. R110-based caspase substrates are more sensitive than coumarin-based caspases substrates (e.g., AMC and AFC), but have narrower dynamic ranges due to the two-step cleavage process. It is recommended that R110-based caspase substrates be used for end point assays while AMC and AFC caspase substrates are used for kinetic assays.

Figure .1 From left to right, the excitation and emission spectra of AMC (7-Amino-4-methylcoumarin), AFC (7-Amino-4-trifluoromethylcoumarin), Rhodamine 110(R110) and ProRed™ 620.
Table 4. Fluorogenic caspase substrates.
| Substrate | Target for Caspases | Ex (nm) | Em (nm) | ε¹ | Φ² | Unit Size | Cat No. |
| Ac-DEVD-AFC *CAS 201608-14-2* | Caspase 3, 7 | 376 | 482 | 17000 | 0.53 | 5 mg | 13401 |
| Ac-DEVD-AMC *CAS 169332-61-0* | Caspase 3, 7 | 341 | 441 | 19000 | N/D | 5 mg | 13402 |
| Z-DEVD-AFC | Caspase 3, 7 | 376 | 482 | 17000 | 0.53 | 5 mg | 13420 |
| Z-DEVD-AMC *CAS 1135416-11-3* | Caspase 3, 7 | 341 | 441 | 19000 | N/D | 5 mg | 13421 |
| Z-DEVD-ProRed™ 620 | Caspase 3, 7 | 532 | 619 | N/D | N/D | 1 mg | 13433 |
| (Z-DEVD)2-R110 *CAS 223538-61-2* | Caspase 3, 7 | 500 | 522 | 80000 | N/D | 1 mg | 13430 |
| Z-DEVD-ProRed™ 620 | Caspase 3, 7 | 532 | 619 | N/D | N/D | 1 mg | 13433 |
| Ac-IETD-AFC *CAS 211990-57-7* | Caspase 8, Granzyme B | 376 | 482 | 17000 | 0.53 | 5 mg | 13410 |
| Ac-IETD-AMC | Caspase 8, Granzyme B | 341 | 441 | 19000 | N/D | 5 mg | 13411 |
| Z-IETD-AFC *CAS 219138-02-0* | Caspase 8, Granzyme B | 376 | 482 | 17000 | 0.53 | 5 mg | 13425 |
| (Ac-IETD)2-R110 | Caspase 8, Granzyme B | 500 | 522 | 80000 | N/D | 1 mg | 13431 |
| Z-IETD-ProRed™ 620 | Caspase 8, Granzyme B | 532 | 619 | N/D | N/D | 1 mg | 13434 |
| Ac-LEHD-AMC *CAS 292633-16-0* | Caspase 9 | 341 | 441 | 19000 | N/D | 5 mg | 13426 |
| (Ac-LEHD)2-R110 | Caspase 9 | 500 | 522 | 80000 | N/D | 1 mg | 13427 |
| Z-LEHD_ProRed™ 620 | Caspase 9 | 532 | 619 | N/D | N/D | 1 mg | 13435 |
Note
- ε = molar extinction coefficient at their maximum absorption wavelength (Units = cm-1M-1).
- Φ = fluorescence quantum yield in aqueous buffer (pH 7.2).
Caspase Inhibitors
Caspase inhibitors bind to the active site of caspases and form either a reversible or irreversible linkage. Generally the structure of caspase inhibitors consist of the caspase recognition sequence and a functional group such as aldehyde (-CHO) or fluoromethylketone (-FMK). Caspase inhibitors with an aldehyde functional group are reversible, whereas inhibitors which have FMK are irreversible. Both caspase substrates and inhibitors have minimal cytotoxic effects and, therefore, are useful tools when studying caspase activity.
Table 5. Reversible and irreversible binding caspase inhibitors.
| Inhibitor | Target for Caspases | Binding | Ex (nm) | Em (nm) | Unit Size | Cat No. |
| Ac-DEVD-CHO *CAS 169332-60-9* | Caspase 3, 7 | Reversible | – | – | 1 mg | 13403 |
| Ac-IETD-CHO *CAS 191338-86-0* | Caspase 8 | Reversible | – | – | 5 mg | 13412 |
| mFluor™ 450-VAD-FMK | Caspase 1, 2, 3, 6, 8, 9, 10 | Irreversible | 406 | 445 | 25 tests | 13475 |
| mFluor™ 510-VAD-FMK | Caspase 1, 2, 3, 6, 8, 9, 10 | Irreversible | 412 | 505 | 25 tests | 13476 |
| FITC-C6-DEVD-FMK | Caspase 3, 7 | Irreversible | 491 | 516 | 1 mg | 13406 |
| FITC-C6-DEVD-FMK | Caspase 3, 7 | Irreversible | 491 | 516 | 100 µg | 13408 |
| FITC-C6-LEHD-FMK | Caspase 9 | Irreversible | 491 | 516 | 1 mg | 13407 |
| FITC-C6-LEHD-FMK | Caspase 9 | Irreversible | 491 | 516 | 100 µg | 13409 |
| FAM-VAD-FMK | Caspase 1, 2, 3, 6, 8, 9, 10 | Irreversible | 493 | 517 | 25 tests | 13470 |
| SRB-VAD-FMK [Sulforhodamine B-VAD-FMK] | Caspase 1, 2, 3, 6, 8, 9, 10 | Irreversible | 559 | 577 | 25 tests | 13472 |
| TF4-VAD-FMK | Caspase 1, 2, 3, 6, 8, 9, 10 | Irreversible | 578 | 602 | 25 tests | 13471 |
Live Cell Caspase Activity Assays
The Cell Meter™ Caspase Activity Assay kits provide a robust and convenient method to measure caspase activity in live cells. These assays utilize cell-permeable substrates containing the recognition sites to measure either caspases 3 and 7, caspase 8, or caspase 9 activity, as well as a multiplex assay designed to simultaneously measure all four caspases. Conjugation of the caspase recognition sequence inhibits the fluorophore or chromophore’s ability to produce a quantifiable signal. In the presence of the appropriate activated caspase, the fluorophore or chromophore is cleaved from the recognition sequence and free to produce either a bright fluorescent or colored signal indicative of apoptosis. The assays are robust and highly specific for their respective activated caspase. Designed to require no-wash steps, Cell Meter™ Caspase Activity Assay kits can be readily adapted to high throughput screening. Using 100 µL of reagents per well in a 96-well format, this kit provides sufficient reagents to perform 200 assays. Using 25 µL of reagents per well in a 384-well format, this kit provides sufficient reagents to perform 800 assays.
Figure 2. Detection of Caspase Activities in Jurkat cells. Jurkat cells were seeded on the same day at 200,000 cells/well in a Costar black wall/clear bottom 96-well plate. The cells were treated with staurosporine at the final concentration of 1 mM for 4 hours (Red Bar) while the untreated cells were used as control (Blue Bar). The single-caspase assay loading solution (100 µL/well) was added (in #1 for caspase 3/7, #2 for caspase 8 or #3 for caspase 9) or Triple-caspase assay loading solution (#4 for caspase 3/7, 8 and 9 together) was added, and incubated at room temperature for 1 hour. The fluorescence intensity was measured with FlexStation fluorescence microplate reader at the indicated wavelength. The caspase 3/7, 8 and 9 activities can be detected in a single assay without interferences from other caspases.
Table 6. Assay kits for measuring Caspase 3, 7, 8 and 9 activity
| Kit | Sample Type | Platform | Ex (nm) | Em (nm) | Unit Size | Cat No. |
| Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Blue Fluorescence* | Live Cells | Microplate, HCS | 360 | 570 | 200 tests | 22795 |
| Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Green Fluorescence* | Live Cells | Microplate, HCS | 490 | 525 | 200 tests | 22796 |
| Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Red Fluorescence* | Live Cells | Microplate, HCS | 540 | 620 | 200 tests | 22797 |
| Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Green Fluorescence Optimized for Flow Cytometry* | Live Cells | Flow Cytometer | 488 | 530 | 100 tests | 22823 |
| Cell Meter™ Caspase 8 Activity Apoptosis Assay Kit *Blue Fluorescence* | Live Cells | Microplate, HCS | 370 | 450 | 200 tests | 22812 |
| Cell Meter™ Caspase 8 Activity Apoptosis Assay Kit *Green Fluorescence* | Live Cells | Microplate, HCS | 490 | 525 | 200 tests | 22798 |
| Cell Meter™ Caspase 8 Activity Apoptosis Assay Kit *Red Fluorescence* | Live Cells | Microplate, HCS | 540 | 620 | 100 tests | 22816 |
| Cell Meter™ Caspase 9 Activity Apoptosis Assay Kit *Blue Fluorescence* | Live Cells | Microplate, HCS | 375 | 435 | 200 tests | 22813 |
| Cell Meter™ Caspase 9 Activity Apoptosis Assay Kit *Green Fluorescence* | Live Cells | Microplate, HCS | 490 | 525 | 200 tests | 22798 |
| Cell Meter™ Caspase 9 Activity Apoptosis Assay Kit *Red Fluorescence* | Live Cells | Microplate, HCS | 540 | 620 | 100 tests | 22816 |
| Cell Meter™ Multiplexing Caspase 3/7, 8 and 9 Activity Assay Kit *Triple Fluorescence Colors* | Live Cells | Microplate, HCS | Triple Fluorescence | Triple Fluorescence | 100 tests | 22820 |
Solution-Based Caspase 3/7 Activity Assays
The Amplite™ Caspase 3/7 Activity Assay kit provides a simple and robust method to measure caspase 3 and 7 in cell extracts and purified enzyme preparations. The assays use substrate containing a DEVD recognition sequence specific for caspase 3/7. Conjugation of the DEVD recognition sequence masks the signal output of the attached fluorophore or chromophore. In the presence of caspase 3/7, the fluorophore or chromophore is cleaved from the DEVD sequence and free to produce either a bright fluorescence or colored signal indicative of apoptosis.
Fluorimetric caspase 3/7 activity assay kits included:
- Caspase 3/7 Substrate (200X Stock Solution)
- Caspase 3/7 Inhibitor
- Assay Buffer
- DTT (Reducing agent)
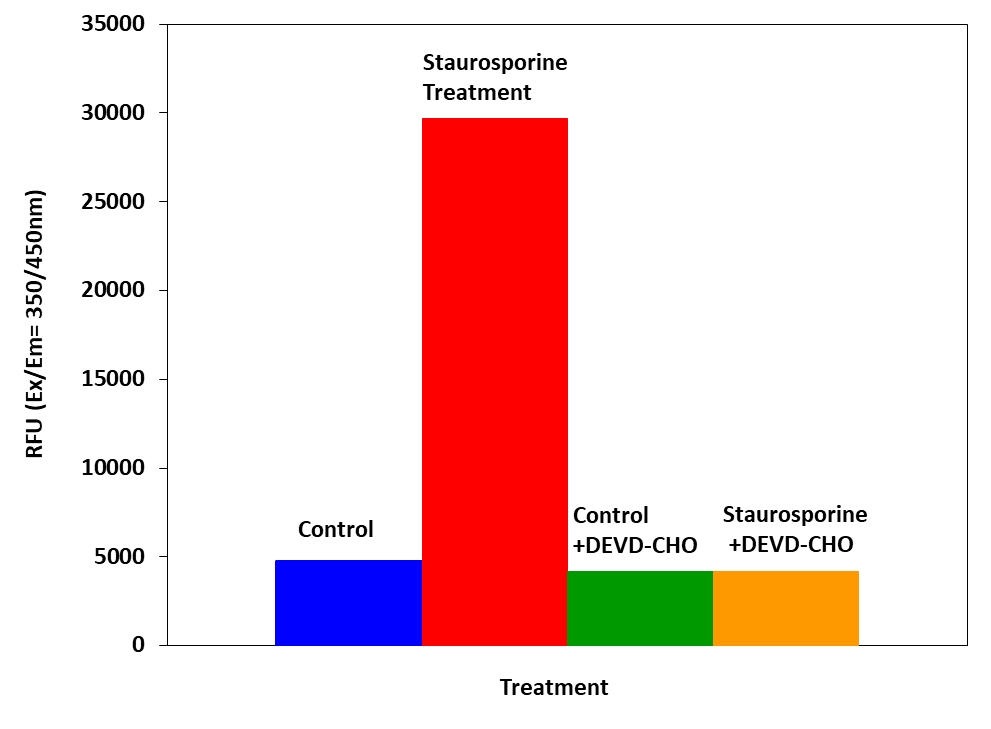
Figure 3. Detection of Caspase 3/7 activity in Jurkat cells with Amplite™ Fluorimetric Caspase 3/7 Assay Kit. Jurkat cells were seeded on the same day at 80,000 cells/well/90 µL in a Costar black wall/clear bottom 96-well plate. The cells were treated with or without 20 µM of camptothecin for 5 hours, and with or without 5 µM of the caspase inhibitor AC-DEVD-CHO for 10 minutes. The caspase 3/7 assay solution (100 µL/well) was added and incubated at room temperature for 1 hour. The fluorescence intensity was measured at Ex/Em = 350/450 nm.
Table 7. Solution-based assays for measuring caspase 3/7 activity.
| Product Name | Sample Type | Platform | Ex (nm) | Em (nm) | Unit Size | Cat No. |
| Amplite™ Colorimetric Caspase 3/7 Assay Kit *Yellow Color* | Cell Extracts | Absorbance Microplate Reader | 490 | None | 200 tests | 13507 |
| Amplite™ Fluorimetric Caspase 3/7 Assay Kit *Blue Fluorescence* | Cell Extracts | Fluorescence Microplate Reader | 350 | 450 | 500 tests | 13502 |
| Amplite™ Fluorimetric Caspase 3/7 Assay Kit *Green Fluorescence* | Cell Extracts | Fluorescence Microplate Reader | 490 | 525 | 500 tests | 13503 |
| Amplite™ Fluorimetric Caspase 3/7 Assay Kit *Red Fluorescence* | Cell Extracts | Fluorescence Microplate Reader | 535 | 620 | 100 tests | 13504 |
Caspase Binding Assays
In the process of apoptosis, one of the key events is the activation of caspases, which is important for the initiation of apoptosis. Cell Meter™ Live Cell Caspase Binding Kits use fluorescent cell permeable and nontoxic indicators to detect caspases 1, 2, 3/7, 6, 8, 9, 10, and 13 activities. Once bound to caspases, the fluorescent reagents are retained inside the cell. The binding event prevents the caspases from further catalysis but will not stop apoptosis from proceeding. The caspase binding kits are applicable for fluorescence microscope, flow cytometer, and fluorescence microplate reader. The kits provide all the essential components with an optimized assay protocol, as well as a Hoechst dye (Ex/Em = 350/461 nm) for labeling the whole population of cells and a propidium iodide dye (Ex/Em = 535/635) or Nuclear Green™ DCS1 (Assay Kit 20101) for staining necrotic cells.
Caspase binding assays kits included:
- Fluorescent Caspase Inhibitor
- 500X Hoechst (Live Cell DNA Stain)
- 500X Propidium Iodide or 500X Nuclear Grean™ DCS1 (Necrotic Cells Stain)
- Washing Buffer
Figure 3. Fluorometric detection of active caspases 3/7 using FAM-DEVD-FMK (Cat No. 20100) in Jurkat cells. The cells were treated with 1 µM staurosporine for 4 hours (Red) while untreated cells were used as a control (Green). Control and treated cells were incubated with FAM-DEVD-FMK for 1 hour at 37 °C, and then washed once after stain. Fluorescent intensity was measured with NovoCyte™ 3000 Flow Cytometer FITC channel.
Table 8. Binding assays for measuring caspase 1, 2, 3/7, 6, 8, 9, 10, or 13 activity.
| Product Name | Target Caspase | Ex (nm) | Em (nm) | Filter Set | Channel | Unit Size | Cat No. |
| Cell Meter™ Live Cell Caspase 1 Binding Assay Kit *Green Fluorescence* | Caspase 1 | 490 | 525 | FITC | FL1 | 25 Tests | 20108 |
| Cell Meter™ Live Cell Caspase 2 Binding Assay Kit *Green Fluorescence* | Caspase 2 | 490 | 525 | FITC | FL1 | 25 Tests | 20111 |
| Cell Meter™ Live Cell Caspase 3/7 Binding Assay Kit *Green Fluorescence* | Caspase 3/7 | 490 | 525 | FITC | FL1 | 25 Tests | 20100 |
| Cell Meter™ Live Cell Caspase 3/7 Binding Assay Kit *Red Fluorescence* | Caspase 3/7 | 550 | 595 | TRITC | FL1 | 25 Tests | 20101 |
| Cell Meter™ Live Cell Caspase 6 Binding Assay Kit *Green Fluorescence* | Caspase 6 | 490 | 525 | FITC | FL1 | 25 Tests | 20113 |
| Cell Meter™ Live Cell Caspase 8 Binding Assay Kit *Green Fluorescence* | Caspase 8 | 490 | 525 | FITC | FL1 | 25 Tests | 20115 |
| Cell Meter™ Live Cell Caspase 9 Binding Assay Kit *Green Fluorescence* | Caspase 9 | 490 | 525 | FITC | FL1 | 25 Tests | 20117 |
| Cell Meter™ Live Cell Caspase 10 Binding Assay Kit *Green Fluorescence* | Caspase 10 | 490 | 525 | FITC | FL1 | 25 Tests | 20119 |
| Cell Meter™ Live Cell Caspase 13 Binding Assay Kit *Green Fluorescence* | Caspase 13 | 490 | 525 | FITC | FL1 | 25 Tests | 20125 |
| Cell Meter™ Generic Fluorimetric Caspase Binding Assay Kit *Red Fluorescence Optimized for Flow Cytometry* | Caspase-1, 3/7, 4, 5, 6, 8, 9 | 649 | 664 | – | APC | 25 Tests | 20125 |
Caspase & Phosphatidylserine Multiplexing Assay
Cell Meter™ Live Cell Caspase 3/7 and Phosphatidylserine Detection Kit is designed to detect apoptosis by simultaneously monitoring Caspase 3/7 and annexin V activities in mammalian cells. Annexins are a family of proteins that bind to phospholipid membranes in the presence of calcium. Annexin V is used to detect apoptotic cells that express phosphatidylserine (PS) on the cell surface. The appearance of PS on the cell surface is a universal indicator of the initial/intermediate stages of cell apoptosis. Annexin V-dye conjugates monitor cell apoptosis through measuring the translocation of PS. The kit also provides a Hoechst dye for labeling the nucleus of the whole population of the cells, and propidium iodide dye for staining necrosis cells.
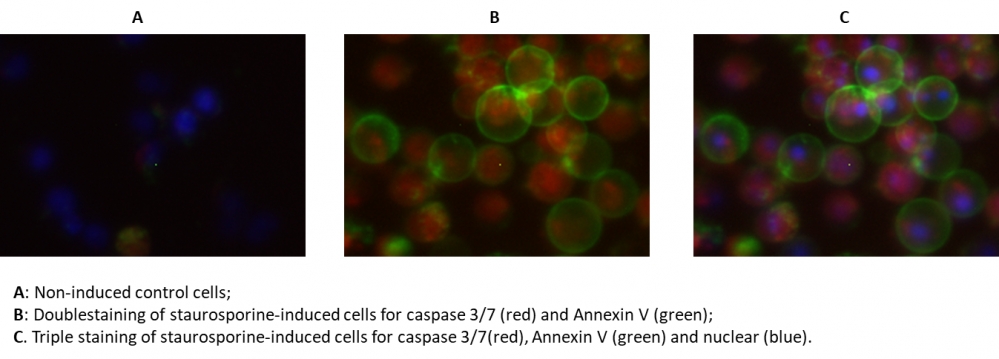
Figure 4. The fluorescence image analysis indicated the increased expression of caspase 3/7 (red, stained by TF3-DEVD-FMK) and annexin V (Green, stained by Annexin V-iFluor 488™) in Jurkat cells induced by 1 µM staurosporine for 3 hour. The fluorescence images of the cells (300,000 cells/well) were taken with Olympus fluorescence microscope through the DAPI, FITC, and TRITC channel respectively. Individual images taken from each channel from the same cell population were merged as shown above. A: Non-induced control cells; B: Doublestaining of staurosporine-induced cells for caspase 3/7 (red) and Annexin V (green); C: Triple staining of staurosporine-induced cells for caspase 3/7 (red), annexin V (green) and nuclear (blue).
Table 9. Caspase and phosphatidylserine detection kit for measuring apoptosis.
| Product Name | Unit Size | Cat No. |
| Cell Meter™ Live Cell Caspase 3/7 and Phosphatidylserine Detection Kit *Triple Fluorescence Colors* | 100 Tests | 22850 |
Table 10. Ordering Info for Caspase Substrates and Inhibitors Products
| Cat# | Product Name | Unit Size |
| 13401 | Ac-DEVD-AFC *CAS 201608-14-2* | 5 mg |
| 13402 | Ac-DEVD-AMC *CAS 169332-61-0* | 5 mg |
| 13403 | Ac-DEVD-CHO *CAS 169332-60-9* | 1 mg |
| 13405 | Ac-DEVD-pNA *CAS 189950-66-1* | 5 mg |
| 13406 | FITC-C6-DEVD-FMK | 1 mg |
| 13407 | FITC-C6-LEHD-FMK | 1 mg |
| 13408 | FITC-C6-DEVD-FMK | 100 ug |
| 13409 | FITC-C6-LEHD-FMK | 100 ug |
| 13410 | Ac-IETD-AFC *CAS 211990-57-7* | 5 mg |
| 13411 | Ac-IETD-AMC | 5 mg |
| 13412 | Ac-IETD-CHO *CAS 191338-86-0* | 5 mg |
| 13413 | Z-IETD-pNA *CAS 219138-21-3* | 5 mg |
| 13420 | Z-DEVD-AFC | 5 mg |
| 13421 | Z-DEVD-AMC | 5 mg |
| 13422 | Z-DEVD-pNA | 5 mg |
| 13425 | Z-IETD-AFC *CAS 219138-02-0* | 5 mg |
| 13426 | Ac-LEHD-AMC *CAS 292633-16-0* | 5 mg |
| 13427 | (Ac-LEHD)2-R110 | 1 mg |
| 13430 | (Z-DEVD)2-R110 | 1 mg |
| 13431 | (Ac-IETD)2-R110 | 1 mg |
| 13433 | Z-DEVD-ProRed™ 620 | 1 mg |
| 13434 | Z-IETD-ProRed™ 620 | 1 mg |
| 13435 | Z-LEHD-ProRed™ 620 | 1 mg |
| 13470 | FAM-VAD-FMK | 25 Tests |
| 13471 | TF4-VAD-FMK | 25 Tests |
| 13472 | SRB-VAD-FMK [Sulforhodamine B-VAD-FMK] | 25 Tests |
| 13473 | 5-FAM-YVAD-FMK | 25 Tests |
| 13474 | TF3-DEVD-FMK | 25 Tests |
| 13475 | mFluor™ Violet 450-VAD-FMK | 25 Tests |
| 13476 | mFluor™ 510-VAD-FMK | 25 Tests |
Table 11. Ordering Info for Caspase Activity and Binding Assays Products
| Cat# | Product Name | Unit Size |
| 13502 | Amplite™ Fluorimetric Caspase 3/7 Assay Kit *Blue Fluorescence* | 500 Tests |
| 13503 | Amplite™ Fluorimetric Caspase 3/7 Assay Kit *Green Fluorescence* | 500 Tests |
| 13504 | Amplite™ Fluorimetric Caspase 3/7 Assay Kit *Red Fluorescence* | 100 tests |
| 13507 | Amplite™ Colorimetric Caspase 3/7 Assay Kit *Yellow Color* | 200 Tests |
| 20100 | Cell Meter™ Live Cell Caspase 3/7 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 20101 | Cell Meter™ Live Cell Caspase 3/7 Binding Assay Kit *Red Fluorescence* | 25 Tests |
| 20108 | Cell Meter™ Live Cell Caspase 1 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 20111 | Cell Meter™ Live Cell Caspase 2 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 20113 | Cell Meter™ Live Cell Caspase 6 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 20115 | Cell Meter™ Live Cell Caspase 8 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 20117 | Cell Meter™ Live Cell Caspase 9 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 20119 | Cell Meter™ Live Cell Caspase 10 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 20125 | Cell Meter™ Live Cell Caspase 13 Binding Assay Kit *Green Fluorescence* | 25 Tests |
| 22795 | Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Blue Fluorescence* | 200 Tests |
| 22796 | Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Green Fluorescence* | 200 Tests |
| 22797 | Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Red Fluorescence* | 100 Tests |
| 22798 | Cell Meter™ Caspase 8 Activity Apoptosis Assay Kit *Green Fluorescence* | 200 Tests |
| 22799 | Cell Meter™ Caspase 9 Activity Apoptosis Assay Kit *Green Fluorescence* | 200 Tests |
| 22812 | Cell Meter™ Caspase 8 Activity Apoptosis Assay Kit *Blue Fluorescence* | 200 tests |
| 22813 | Cell Meter™ Caspase 9 Activity Apoptosis Assay Kit *Blue Fluorescence* | 200 Tests |
| 22816 | Cell Meter™ Caspase 8 Activity Apoptosis Assay Kit *Red Fluorescence* | 100 Tests |
| 22817 | Cell Meter™ Caspase 9 Activity Apoptosis Assay Kit *Red Fluorescence* | 100 Tests |
| 22820 | Cell Meter™ Multiplexing Caspase 3/7, 8 and 9 Activity Assay Kit *Triple Fluorescence Colors* | 100 Tests |
| 22821 | Cell Meter™ Generic Fluorimetric Caspase Activity Assay Kit *Green Fluorescence Optimized for Flow Cytometry* | 100 Tests |
| 22822 | Cell Meter™ Generic Fluorimetric Caspase Binding Assay Kit *Red Fluorescence Optimized for Flow Cytometry* | 100 Tests |
| 22823 | Cell Meter™ Caspase 3/7 Activity Apoptosis Assay Kit *Green Fluorescence Optimized for Flow Cytometry* | 100 Tests |
| 22850 | Cell Meter™ Live Cell Caspase 3/7 and Phosphatidylserine Detection Kit *Triple Fluorescence Colors* | 100 Tests |
Internucleosomal DNA fragmentation caused by activated endonucleases is universally regarded as the biochemical hallmark of apoptosis. Cells containing these DNA strand breaks can be identified with the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay. This well-established in situ staining method relies on the enzyme terminal deoxynucleotidyl transferase (TdT) to catalyze the incorporation of modified dUTPs to the 3′-hydroxyl termini of fragmented DNA. Depending upon the choice of modified dUTP – BrdUTP, biotin-dUTP or a fluorescein-dUTP – apoptotic cells can be identified and measured using a variety of different detection strategies and systems, including fluorescence microscopy, flow cytometry and fluorescence-based microplate studies.
AAT Bioquest offers a number of fluorimetric TUNEL assays optimized for in situ apoptosis detection in live cells or fixed cells and tissue samples. These products can be combined with other cell-based viability and apoptosis assays to provide a comprehensive assessment of cell health, assess toxicity and safety of drug candidates, or analyze cancer and disease related cellular changes.
TUNEL Assay Principles
During the later-stages of apoptosis, caspase-activated endonucleases cleave genomic DNA into oligonucleosomal fragments (∼ 180-200 base pairs long). Using the TUNEL assay, the exposed 3′-OH termini of these breaks can be marked with modified dUTPs for subsequent visualization and quantification of apoptotic cells in situ. This is generally done either directly using dye-modified dUTP or indirectly using BrdUTP and antibody conjugates against BrdUTP. Regardless of the labeling strategy, both require the enzyme terminal deoxynucleotidyl transferase (TdT) to catalyze the incorporation of the modified dUTPs to the 3′-OH termini.
Detection of DNA fragments by this assay requires sample fixation using a crosslinking reagent such as 4% paraformaldehyde (avoid ethanol-based fixatives as it hinders the extraction of small DNA fragments) and sample permeabilization. Both steps are critical to the TUNEL assay as it facilitates the entry of exogenous TdT and antibody conjugates against BrdUTP. Keep in mind that several variables influence the staining kinetics of the TUNEL assay. Some of these variables, which include reagent concentration, fixation of sample, and accessibility of DNA strand breaks, may vary between tissue or cell sample types. By standardizing the TUNEL assay using samples with positive and negative controls of apoptosis it can alleviate these potential influences and reduce false positives or negative results.

TUNEL Assays for Live Cells
Traditionally the TUNEL assay is performed on fixed and permeabilized cells and tissue samples in order to facilitate the entry of exogenous TdT, which is necessary for labelling. This initial handling of the samples is critical to the outcome, and ultimately requires additional hands on-time in order to optimize conditions so results are accurate and reproducible. The Cell Meter™ Live Cell TUNEL Apoptosis Assays are designed to identify DNA fragmentation without the enzyme TdT and the additional handling that comes with its use. These assays utilize a proprietary membrane-permeant fluorescent dye that passively enters live cells and selectively targets the nicks in DNA that form during apoptosis. The fluorescently-labeled DNA fragments can then be visualized directly by fluorescence microscopy, flow cytometry or fluorescence-based microplate assays. The Cell Meter™ Live Cell TUNEL Apoptosis assays are available in two emission colors (green and red) for flexibility in multiparametric analysis with other fluorescence-based cell function assays, such as caspase activity assays and annexin V staining assays.



Figure 2. Fluorescence images of TUNEL reaction in HeLa cells with the treatment of 100 nM or 1 µM staurosporine (SS) for 4 hours as compare to untreated control. Cells were incubated with TUNEL working solution for 1 hour at 37°C. The red fluorescence signal was analyzed using fluorescence microscope with a TRITC filter set. Fluorescently labeled DNA strand breaks shows intense fluorescent staining in SS treated cells.
Table 1. Cell Meter™ TUNEL assays for live cell analysis
| Assay | Ex/Em (nm)¹ | Cutoff (nm)¹ | Filter Set² | Channel³ | Unit Size | Cat No. |
| Cell Meter™ Live Cell TUNEL Apoptosis Assay Kit *Green Fluorescence* | 490/525 | 515 | FITC Filter Set | FITC Channel | 25 tests | 22849 |
| Cell Meter™ Live Cell TUNEL Apoptosis Assay Kit *Red Fluorescence* | 550/590-650 | 570 | TRITC Filter Set | PE-Cy5 Channel | 25 tests | 22844 |
Note
- Fluorescence microplate instrument specifications.
- Fluorescence microscope instrument specifications.
- Flow cytometer instrument specifications.
TUNEL Assays for Fixed Cells and Tissue Samples
The Cell Meter™ Fixed Cell and Tissue TUNEL Assays follow the traditional TUNEL method and are optimized for in situ apoptosis detection in both fixed cells and tissue sections. These assays directly identify apoptotic cells within a population by using TdT to catalyze the incorporation of dye-modified dUTPs at the 3′-OH termini of fragmented DNA. The fluorescently-labeled DNA fragments can then be visualized or quantified using fluorescence microscopy or flow cytometry, respectively. The Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis assays are available in four emission colors (blue, green, red and deep red) for flexibility in multiparametric analysis with other fluorescent proteins or conjugates, such as phalloidin.
Key Features
- Validated for apoptosis detection in fixed cells and formalin-fixed, paraffin-embedded tissue sections
- Direct incorporation minimizes hands on-time be reducing the number of incubation steps
- Compatible with other fluorescent proteins and conjugates for multiplex analysis
- Assay provides sufficient reagents for 25 tests


Figure 2. TUNEL assay of formalin-fixed paraffin-embedded (FFPE) human lung adenocarcinoma sections with Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Green Fluorescence*. DNA strand breaks showed intense fluorescent staining in DNAse treated tissue section. Cell nucleus was stained with Nuclear Blue™ DCS1 (Cat No. 17548).
Table 2. Cell Meter™ TUNEL assays for fixed cells and tissue analysis
| Assay | dUTP | Ex/Em (nm)¹ | Channel¹ | Filter Set² | Unit Size | Cat No. |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Blue Fluorescence* | DEAC-dUTP | 405/525 | Pacific Orange Channel | Violet Filter Set | 25 tests | 22857 |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Green Fluorescence* | Fluorescein-12-dUTP | 488/530 | FITC Channel | FITC Filter Set | 25 tests | 22851 |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Red Fluorescence* | TMR-dUTP | 488/575 | PE Channel | Cy3/TRITC Filter Set | 25 tests | 22853 |
| Cell Meter™ Fixed Cell and Tissue TUNEL Apoptosis Assay Kit *Deep Red Fluorescence* | TF5-dUTP | 633 or 640/660 | APC Channel | Cy5 Filter Set | 25 tests | 22855 |
Note
- Flow cytometer instrument specifications.
- Fluorescence microscope instrument specifications.
The mitochondrial membrane potential (ΔΨm), generated by the electron transport chain, is a key parameter necessary for healthy mitochondrial functioning. Together with the proton gradient, it generates the driving force behind mitochondrial ATP synthesis. It plays a key role in mitochondrial homeostasis through selective elimination of dysfunctional mitochondria, and is an essential component of mitochondrial calcium homeostasis.
A distinctive feature of the early stages of apoptosis is the disruption of normal mitochondrial function. A collapse in mitochondrial membrane and redox potential may induce unwanted loss of cell viability and be a cause of various pathologies. We offer a wide assortment of fluorescent probes for analyzing aspects of normal mitochondrial activity in live cells, including reactive oxygen species (ROS) production, mitochondrial membrane potential and calcium flux.
JC-10™ Dual-Emission ΔΨm Probe
JC-10™, a derivative of JC-1, is potential-dependent probe used to determine ΔΨm by flow cytometry, fluorescence microscopy and in microplate-based fluorescent assays. In healthy cells, JC-10™ selectively accumulates in mitochondria generating orange J-aggregates that exhibit a broad excitation spectrum and emission maximum at 590 nm. However, in apoptotic and necrotic cells with low ΔΨm, JC-10™ diffuses out of the mitochondria and JC-10™ monomers are generated, resulting in a shift to green emission (525 nm). JC-10™ allows both qualitative and quantitative visualization of ΔΨm changes, considering the shift from orange to green fluorescence emission and the fluorescence intensity ratio, respectively. It has been successfully used as an ΔΨm indicator in a variety of sample types including myocytes, neurons, intacts tissues and isolated mitochondria.
Features of JC-10™:
- Easy-to-Use: JC-10™ does not percipitate when diluted into aqueous buffers, eliminating artifacts.
- Robust: JC-10™ has smaller assay deviations due to its enhanced solubility in aqueous media and higher sensitivity.
- Enhanced Signal: JC-10™ has a higher signal-to-background ratio than JC-1.
- Enhanced Sensitivity: JC-10™ has the ability to detect subtle changes in ΔΨm loss better than JC-1 in all tested cell lines.
- Broad Applications: JC-10™ can be used for primary rat hepatocytes.
- Convenient: JC-10™ is compatible with fluorescence microplate readers, cell imagers and flow cytometers.
Figure 3. Camptothecin induced mitochondrial membrane potential changes were measured with JC-10™ (Cat No. 22204) and JC-1 (Cat No. 22200) in Jurkat cells. After Jurkat cells were treated with camptothecin (10 µM) for 4 hours, JC-1 and JC-10™ dye loading solutions were added to the wells and incubated for 30 minutes. The fluorescent intensities for both J-aggregates and monomeric forms of JC-1 and JC-10™ were measured at Ex/Em = 490/525 nm and 540/590 nm with NOVOstar microplate reader (BMG Labtech).
Table 1. JC-10™ and JC-1 dual-emission mitochondrial membrane potential probes.
| Probe | Ex/Em | Ex/Em | Filter Set | Unit Size | Cat No. |
| JC-10™ *Superior alternative to JC-1* | 508/524 (monomer) | 508/570 (aggregate) | FITC (monomer) TRITC (aggregate) | 5×100 µL | 22204 |
| JC-1 | 515/530 (monomer) | 515/590 (aggregate) | FITC (monomer) TRITC (aggregate) | 5 mg | 22200 |
| JC-1 | 515/530 (monomer) | 515/590 (aggregate) | FITC (monomer) TRITC (aggregate) | 50 mg | 22201 |
Table 2. Cell Meter™ JC-10™ assay kits for measuring mitochondrial membrane potential.
| Probe | Instrument | Ex (nm) | Em (nm) | Cutoff/Channel | Unit Size | Cat No. |
| Cell Meter™ JC-10 Mitochondrion Membrane Potential Assay Kit *Optimized for Microplate Assays* | Microplate Reader | 490/540 nm | 525/590 nm | 515/570 nm | 500 tests | 22800 |
| Cell Meter™ JC-10 Mitochondrion Membrane Potential Assay Kit *Optimized for Flow Cytometry Assays* | Flow Cytometer | 488 nm laser | 530/30 575/26 | FITC Channel PE Channel | 100 tests | 22801 |
Mitochondrial-Selective Rhodamine Esters
Cell-permeable cationic rhodamines, such TMRE and TMRM, are readily sequestered by active mitochondria, and commonly used to label mitochondria in living cells. Like JC-10™, TMRE and TMRM uptake in mitochondria is driven by the mitochondrial membrane potential. Both dyes have been successfully for dymanic and in situ quantitative measurements, to screen for inhibitors of the mitochondrial transition pore, to assess the functionality of mitochondria in living cells, and can be used to discrimate between viable and non-viable cell populations. These potentiometric dyes exhibit minimal self-quenching, low cytotoxicity and have reasonable photostability, and their fluorescence intensities can be measured with either a flow cytometer or fluorescence microscope. In comparison to TMRM, TMRE is slightly more hydrophobic.
Table 3. Potentiometric dyes for measuring mitochondrial membrane potential.
| Probe | Ex (nm) | Em (nm) | Filter Set | Unit Size | Cat No. |
| TMRE [Tetramethylrhodamine ethyl ester] *CAS#: 115532-52-0* | 552 | 574 | TRITC | 25 mg | 22220 |
| TMRM [Tetramethylrhodamine methyl ester] *CAS#: 115532-50-8* | 552 | 574 | TRITC | 25 mg | 22221 |
Product ordering information
Table 4. Potentiometric dyes and kits for measuring mitochondrial membrane potential.
| Probe | Unit Size | Cat No. |
| JC-10 *Superior alternative to JC-1* | 5×100 µL | 22204 |
| JC-1 [5,5,6,6-Tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide] *CAS#: 3520-43-2* | 5 mg | 22200 |
| JC-1 [5,5,6,6-Tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide] *CAS#: 3520-43-2* | 50 mg | 22201 |
| Cell Meter™ JC-10 Mitochondrion Membrane Potential Assay Kit *Optimized for Microplate Assays* | 500 tests | 22800 |
| Cell Meter™ JC-10 Mitochondrion Membrane Potential Assay Kit *Optimized for Flow Cytometry Assays* | 100 tests | 22801 |
| TMRE [Tetramethylrhodamine ethyl ester] *CAS#: 115532-52-0* | 25 mg | 22220 |
| TMRM [Tetramethylrhodamine methyl ester] *CAS#: 115532-50-8* | 25 mg | 22221 |
Glutathione (GSH) is a tripeptide comprised of three amino acids L-cysteine, L-glutamic acid and glycine. It is involved in the development and maintenance of protein disulfide bonds , the transport of amino acids across cell membranes, and in detoxification. Since glutathione contains a thiol group, it serves as a major endogenous antioxidants in cells preventing damage by neutralizing reactive oxygen species such as free radicals and peroxides. Monitoring reduced and oxidized glutathione in biological samples is a useful tool for evaluating the redox and detoxification status of cells and tissues against oxidative and free radical mediated cell injury. While few reagents and assay kits are commercially available for quantifying glutathione, they are hindered by their lack of sensitivity and tedious protocols. Amplite™ Fluorimetric Glutathione assay offers a convenient and ultra-sensitive method for the quantification of glutathione in biological samples.
Amplite™ Fluorimetric Glutathione Assay
Amplite™ Fluorimetric Glutathione assay is a sensitive, one-step fluorimetric method to detect as little as 1 picomole of GSH in a 100 µL assay volume. This assay employs a proprietary non-fluorescent and pH-dependent glutathione sensor that becomes strongly fluorescent upon reacting with thiol. The assay can be performed in a convenient 96-well or 384-well microtiter-plate format and is easily adaptable for automation without a separation step. The fluorescence signal can be easily detected using a fluorescence microplate reader at Ex/Em = 490/520 nm.
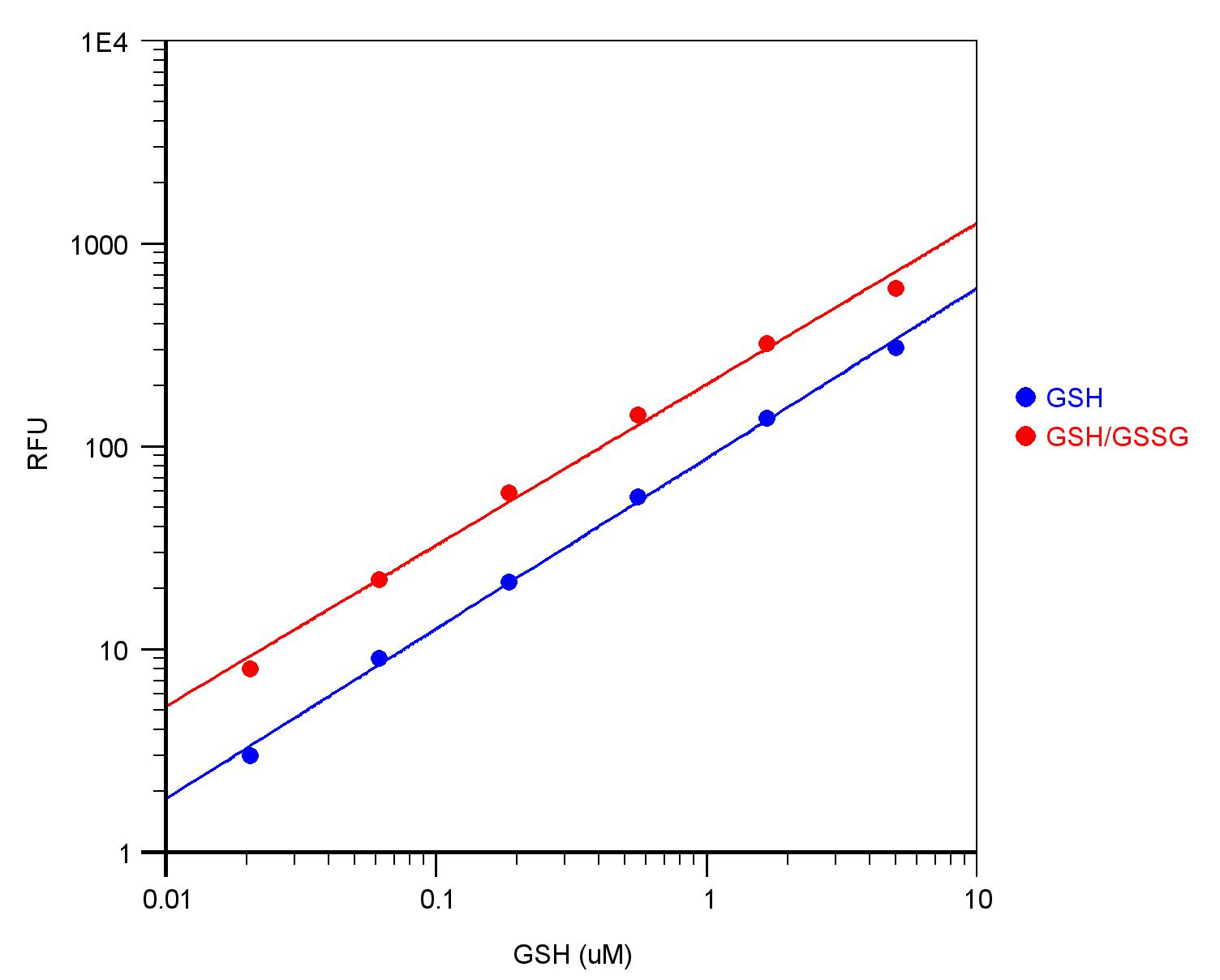
Figure 1. GSH dose responses were measured with Amplite™ Fluorimetric Glutathione Assay Kit (Cat# 10055) on a 96-well black solid plate. As low as 10 nM (1 pmol/well) GSH was detected with 10 minutes incubation.
Glutathione GSH/GSSG Ratio
In healthy cells and tissues, glutathione exists in two states, reduced (GSH) and oxidized (GSSG). In the reduced state, GSH is a major tissue antioxidant that neutralizes reactive oxygen species. As a result of donating an electron, GSH itself becomes reactive and will readily react with other reactive GSH molecules to form GSSG. Additionally, reduced GSH provides reducing equivalents for the glutathione peroxidase (GPx) catalyzed reduction of lipid hydroperoxides to their corresponding alcohols and hydrogen peroxide to water. In the GPx catalyzed reaction, the formation of a disulfide bond between two GSH molecules forms GSSG. The enzyme glutathione reductase (GR) recycles GSSG to GSH while simultaneously oxidizing ?-nicotinamide adenine dinucleotide phosphate (?-NADPH2).
Monitoring reduced GSH and its ratio with oxidized GSSG may be used to evaluate the redox and detoxification status of cells and tissues in relation to the protective role of glutathione against oxidative and free-radical-mediated cell injury. Under normal conditions, the GSH redox couple in mammalian cells typically ranges in concentration from 1 – 10 nM, with resting cells having molar GSH:GSSG ratios exceeding 100:1. When cells are exposed to increased levels of oxidative stress, GSSG accumulates and the ratio of GSH/GSSG decreases to ratios as low as 10:1 and even 1:1. As such, monitoring GSH/GSSG levels in biological samples has become a useful tool in evaluating oxidative stress of cells and tissues, and cell injuries caused by free radicals.
Amplite™ Glutathione GSH/GSSG Ratio Assays
AAT Bioquest Amplite™ Fluorimetric Glutathione GSH/GSSG Ratio Assay and Amplite™ Rapid Fluorimetric Glutathione GSH/GSSG Ratio Assay kits are simple and ultrasensitive assays to quantify as little as 1 picomole of GSH (or cysteine) in a 100 µL assay volume. Each kit uses a proprietary non-fluorescent sensor that becomes strongly fluorescent upon reacting with GSH, and its signal can be detected using a fluorescence microplate reader at Ex/Em = 490/520 nm. Assays can be performed in a convenient 96-well or 384-well microtiter-plate format and is easily adaptable for automation without a separation step. What differs between these assays is the sensor’s solubility. Amplite™ Fluorimetric Glutathione GSH/GSSG Ratio assay uses sensor Thiolite™ Green which is soluble in DMSO. Amplite™ Rapid Fluorimetric Glutathione GSH/GSSG Ratio assay uses a water-soluble sensor Thiolite™ Green 520WS.
Table 1. Ordering Info for Amplite Glutathione Kits (P.O.T) Products
| Cat# | Product Name | Unit Size |
| 10055 | Amplite™ Fluorimetric Glutathione Assay Kit *Green Fluorescence* | 200 Tests |
| 10056 | Amplite™ Fluorimetric Glutathione GSH/GSSG Ratio Assay Kit *Green Fluorescence* | 200 Tests |
| 10060 | Amplite™ Rapid Fluorimetric Glutathione GSH/GSSG Ratio Assay Kit *Green Fluorescence* | 200 Tests |