The biotin-streptavidin complex is one of the most popular tagging systems for the conjugation of biomolecules, such as proteins, lipids and nucleic acids, as well as that of synthetic molecules, such as fluorescent labels. It has found strong success in the area of sample preparation as a core part of many purification systems and plays a critical role in many detection systems for instruments such as microscopy and flow cytometry.
Biotin and Streptavidin
Biotin-Streptavidin Conjugation System
The widespread adoption of the biotin-streptavidin conjugation system is primarily due to two factors. The first is the relatively small size of the biotin and streptavidin molecules themselves, which allows for extensive binding to biologically active macromolecules, such as antibodies, without impedance to their functions (i.e. an antibody’s binding site). The second is the specificity with which biotin and streptavidin bind to each other, as well as the strength of the subsequent bond, which allows for powerful, stream-lined bioassay applications. Streptavidin tetramers have an extraordinarily high binding affinity for biotin with a dissociation constant (Kd) of approximately ∼10-14 mol/L. This tight and specific binding is rapid and able to withstand extremes in pH, temperature, organic solvents, and denaturing reagents.

Figure 1. Illustration of the Streptavidin-Biotin interaction. The multivalent properties of streptavidin allow it to bind up to four biotin molecules with a high degree of affinity. Biotin is typically conjugated to an enzyme, antibody or target protein.
Advantages of the biotin-streptavidin system
A key benefit of the biotin-streptavidin system is its ability to improve detection sensitivity. This in large part is due to the tetrameric conformation of streptavidin. One streptavidin protein has the capacity to bind four biotin molecules with high affinity and selectivity. This multiplicity enables the amplification of weak signals and improves the detection sensitivity for medium- and low-abundance targets in mammalian cells or tissues with a simple workflow.
Another key benefit is the versatility of the biotin-streptavidin system. Because streptavidin can be conjugated to a variety of reporter tags, it can be readily incorporated into virtually every immunoassay. For example, enzyme conjugates of streptavidin are widley used in enzyme-linked immunosorbent assays (ELISAs), while fluorescently labeled streptavidin, such as iFluor™ 488 streptavidin, are widely used in cell surface labeling, fluorescence activated cell sorting (FACS) and other fluorscence imaging applications.
Applications
- Enzyme-linked immunosorbent assay (ELISA)
- Immunohistochemistry (IHC)
- Power Styramide™ Signal Amplification, a superior replacement for tyramide
- Immunoblotting
- Immunofluorescence microscopy
- Cell surface labeling
- Affinity purification
- Fluorescence-activated cell sorting (FACS)
- Flow cytometry
Biotin
Biotin is a small 244 dalton hapten molecule. It’s exceptionally strong binding affinity for streptavidin is commonly exploited to detect and monitor biological targets of interest. Biotin exhibits two characteristics that make it ideal for bioconjugate development. First, is biotin’s relatively small size. This permits the labeling of multiple biotin tags to a single protein or antibody without significantly impeding their biological reactivity. Second, is the valeric acid side chain of the biotin molecule. This chain can be derivatized to facilitate the incorporation of various reactive groups used to chemically label biotin to proteins without altering biotin”s binding affinity for streptavidin.
AAT Bioquest offers biotinylated secondary antibodies, proteins, nucleotides and other small molecules for use in streptavidin-based amplification techniques. For assays in which a biotinylated probe is not available, we provide many biotinylation reagents and kits that enable reasearcher to chemically label proteins, nucleic acids and surface materials to make custom biotinylated reagents.
Featured Products
- Fluorescent biotin derivatives
- Biotinylated secondaries
- Biotinylated nucleotides
- Biotinylated amino acids
- Reactive-biotin derivaitves
- ReadiLink™ Protein Biotinylation Kit
- Amplite™ Colorimetric Biotin Quantitation Kit
Fluorescent Biotin Derivatives
Fluorescent biotin derivatives contains both a fluorophore and a biotin moeity in the same molecule. These reagents are used for detecting and quantiting biotin-binding proteins by fluorescence. The strong quenching associated with streptavidin binding to fluorecent biotin can be used to precisely measure the concentration of streptavidin (or avidin).
Table 1. Available Products for Fluorescent Biotin Derivatives
| Product | Ex/Em (nm) | Ext. Coeff.¹ | FQY² | Unit Size | Cat No |
| Fluorescein biotin | 497/516 | 80,000 | 0.79 | 5 mg | 3017 |
| Biotin-4-fluorescein *CAS 1032732-74-3* | 492/518 | 80,000 | 0.79 | 5 mg | 3006 |
| Cy5 biotin conjugate | 650/669 | 250,000 | 0.27 | 5 mg | 3100 |
Note
- Ext. Coeff. = molar extinction coefficient at their maximum absorption wavelength (Units = cm-1M-1).
- FQY = fluorescence quantum yield in aqueous buffer (pH 7.2).
Biotinylated Secondary Antibodies
We offer biotinylated secondary antibodies to use with streptavidin-based amplification techniques. A key advantage to using a biotinylated secondary is the flexibility to use the same secondary antibody across multiple applications, by simply using a different streptavidin conjugate.
Readily available streptavidin conjugates
- HRP-streptavidin (imaging requires an HRP substrate)
- AP-streptavidin (imaging requires an AP substrate)
- iFluor™-streptavidin
- mFluor™-streptavidin
- APC, PE & tandem dye-streptavidins (The intense bright fluorescence and rapid photobleaching of phycobiliproteins are excellent for flow cytometry applications)
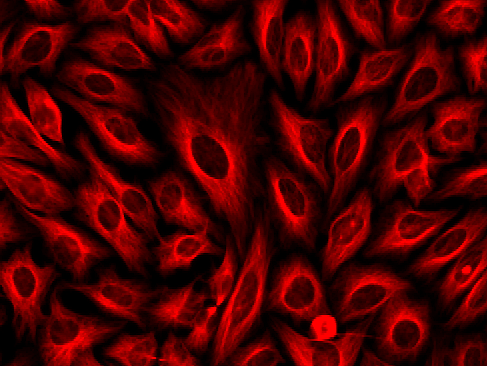
Figure 2. Immunofluorescent stain of α-tubulin in HeLa cells. α-tubulin in fixed and permeabilized HeLa cells were labeled with rabbit anti-tubulin primary antibody, followed by incubation with biotinylated goat anti-rabbit IgG (H&L) (Cat No. 16794), and then visualized with iFluor™ 555-streptavidin conjugate (Cat No. 16989).
Table 2. Available Products for Biotinylated Secondary Antibodies
Biotinylated Nucleotides
Biotin-modified nucleoside triphosphate analogs, such as dUTP and dCTP, can be enzymatically incorparted into DNA or RNA fragments for use in fluorescence in situ hybridization (FISH), DNA arrays, microarrays and other hybridization techniques. Standard enzymatic non-radioactive DNA labeling reactions including 3”-end labeling, cDNA labeling, nick translation, PCR and random prime labeling. Each biotinylated nucleotide contains either an 11-, 14-, 16- or 20-atom spacer between the biotin and its attachment point on the nulceotide. This facilitates its detection and signal amplification by fluorophore and enzyme streptavidin conjugates or agarose and magnetic beads.
Biotinylated Amino Acids
Biotinylated peptides are suitable for immunodetection studies and biomedical screening assays that require immobilization onto streptavidin or avidin coated microtitre plates, beads or membranes. Biotin can be linked to the N-terminus of peptides or to the side chains of lysine (Lys), glutamic acid (Glu) or aspartic acid (Asp) during Fmoc solid phase synthesis. Incorportion of a PEG-spacer in both FMOC-Glu(biotinyl-PEG)-OH and FMOC-Asp(biotinyl-PEG)-OH reduces steric hindrance between target peptide and streptavidin, leading to better biotin binding. The hydrophilic nature of the PEG-spacer minimizes non-specific interactions and improves the solubility of the reagent and biotinylated peptide.
Table 4. Available Biotinylated Amino Acids
| Product | Labeling Principle | Derivative | Unit Size | Cat No. |
| FMOC-Lys(Biotin)-OH | Uses FMOC-SPPS to prepare biotin-labeled peptides | Lysine | 100 mg | 5020 |
| FMOC-Glu(biotinyl-PEG)-OH | Uses FMOC-SPPS to prepare biotin-labeled peptides | Glutamic acid | 100 mg | 5026 |
| FMOC-Glu(biotinyl-PEG)-OH | Uses FMOC-SPPS to prepare biotin-labeled peptides | Glutamic acid | 1 g | 5027 |
| FMOC-Asp(biotinyl-PEG)-OH | Uses FMOC-SPPS to prepare biotin-labeled peptides | Aspartic acid | 100 mg | 5023 |
| FMOC-Asp(biotinyl-PEG)-OH | Uses FMOC-SPPS to prepare biotin-labeled peptides | Aspartic acid | 1 g | 5024 |
Reactive-Biotin Derivatives
Reactive biotin derivatives contain reactive moieties, such as succinimidyl esters or maleimides, that facilitate the labeling of biotin onto proteins, antibodies and other macromolecules. When selecting a reactive biotin derivative, consider the following:
- Funtional group targeting – specific reactive moieties target and bind to their respective functional groups for targeted or non-selective biotinylation of proteins and other macromolecules (e.g. succinimidyl esters target primary amines and maleimides target thiol or sulfhydryl groups).
- Solubility – this influences biotinylation of the target protein or macromolecule.
- Spacer Arm Length (PEG) – enhance detection senstivity of the target protein and reduces steric hindrance
Table 5. Available Reactive Biotinylation Reagents
| Product | Chemical Reactivity | Reactive Moiety | Unit Size | Cat No. |
| Biotin, succinimidyl ester *CAS 35013-72-0* | Amine | Succinimidyl Ester | 100 mg | 3002 |
| Biotin-X, succinimidyl ester *CAS 72040-63-2* | Amine | Succinimidyl Ester | 25 mg | 3010 |
| Biotin PEG2 succinimidyl ester | Amine | Succinimidyl Ester | 25 mg | 3016 |
| Biotin PEG4 succinimidyl ester | Amine | Succinimidyl Ester | 25 mg | 3022 |
| Biotin C2 maleimide | Thiol | Maleimide | 25 mg | 3005 |
| Biotin PEG2 maleimide *CAS 305372-39-8* | Thiol | Maleimide | 5 mg | 3015 |
| Biotin PEG2 amine *CAS 138529-46-1* | Aldehyde, Carboxylic Acid, Ketone | Amine | 5 mg | 3014 |
| Biotin PEG3 amine | Aldehyde, Carboxylic Acid, Ketone | Amine | 5 mg | 3024 |
| Biotin cadaverine | Aldehyde, Carboxylic Acid, Ketone | Cadaverine | 5 mg | 3004 |
| Biotin ethylenediamine *CAS 216299-38-6* | Aldehyde, Carboxylic Acid, Ketone | Ethylenediamine | 5 mg | 3003 |
| Biotin hydrazide *CAS 66640-86-6* | Aldehyde, Carboxylic Acid, Ketone | Hydrazide | 5 mg | 3007 |
| Biotin Azide | Alkyne | Azide | 5 mg | 3020 |
| Biotin-PEG3-azide *CAS 945633-30-7* | Alkyne | Azide | 5 mg | 3019 |
| Biotin Alkyne *CAS 1006592-45-5* | Azide | Alkyne | 5 mg | 3021 |
ReadiView™ Biotinylation Reagents
Determining a protein’s degree of biotinylation is quite challenging because the intrinsic absorption of biotin is difficult to discern from proteins and nucliec acids. To facilitate this determination, we offer a series of chromophoric reactive boitin derivatives, ReadiView™ biotin. These novel biotinylation reagents contain a specially designed ‘Color Tag (CT)’ optimally positioned between two spacer arms. These spacer arms reduce steric hindrance and improve water solubility, while the CT tag makes the degree of biotinylation readily quantifiable by calculating the absorption ratio of 280 nm/385 nm. The CT tag does not affect biotins complexation with streptavidin and has minimal quenching affect on fluorescent streptavidin conjugates. ReadiView™ biotin is availble in amine-reactive, thiol-reactive and click-chemistry formats.
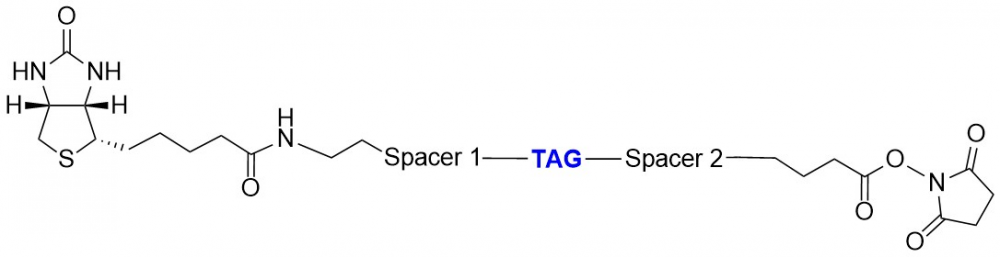
Figure 3. ReadiView™ biotin succinimidyl ester (Cat No. 3059) conjugated with specially designed color tag (CT) for easy determination of biotinylation.
Table 6. Available ReadiView™ Biotin Products
| Product | Chemical Reactivity | Spacer | Unit Size | Cat No. |
| ReadiView™ biotin succinimidyl ester | Amines | Pegylated | 5 mg | 3059 |
| ReadiView™ biotin maleimide | Thiol or sulfhydryl groups | Pegylated | 5 mg | 3058 |
| ReadiView™ biotin hydrazide | Aldehyde, Carboxylic acid, Ketone | Pegylated | 5 mg | 3055 |
| ReadiView™ biotin amine | Carbonyl | Pegylated | 5 mg | 3053 |
| ReadiView™ biotin acid | Amine | Pegylated | 5 mg | 3050 |
Enzyme-Labeled Streptavidin Conjugates
Enzyme-labeled streptavidin conjugates are commonly used in enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, immunoblotting, and in situ hybridization techniques. Enzymes horseradish peroxidase (HRP) and alkaline phosphatase (ALP) are extensively used as reporter tags because of their high turnover rate, stability, ease of conjugation and low cost.
To maximize retention of both enzyme and protein activity, our HRP-streptavidin and AP-streptavidin conjugates are prepared at a 1:1 (enzyme to streptavidin) labeling ratio. Chromogenic, fluorogenic and chemiluminescent stubstrates are available for these enzyme conjugates.
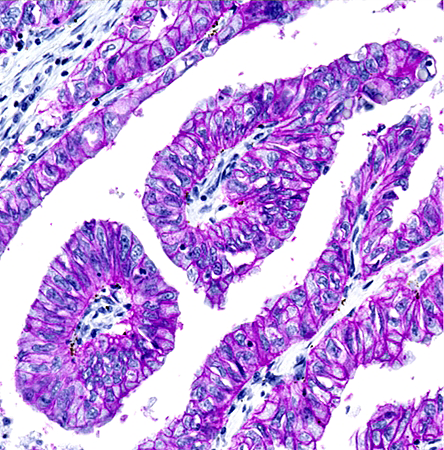
Figure 4. IHC detection of EpCAM in FFPE lung adenocarcinoma tissue. Human adenocarcinoma tissue sections were incubated with rabbit anti-EpCAM primary antibody followed by biotyinylated goat-anti rabbit IgG (H&L). The samples were then incubated with HRP-streptavidin, and the signal was developed using ReadiUse™ Stayright™ Purple.
Table 7. Available Enzyme-labeled Streptavidin Conjugates
| Product | Enzyme | Unit Size | Cat No. |
| HRP-streptavidin conjugate | Horseradish peroxidase (HRP) | 1 mg | 16920 |
| AP-streptavidin conjugate | Alkaline phosphatase (AP) | 1 mg | 16921 |
Table 8. Available HRP Substrates
| Product | Detection | Abs/Ex (nm) | Ex (nm) | Unit Size | Cat No. |
| ReadiUse™ ABTS | Chromogenic | 420 | 1 L | 11001 | |
| ReadiUse™ TMB | Chromogenic | 650 | 100 mL | 11012 | |
| ReadiUse™ TMB | Chromogenic | 650 | 1 L | 11003 | |
| Amplite™ ADHP | Fluorogenic | 571 | 584 | 25 mg | 11000 |
| Amplite™ Blue | Fluorogenic | 324 | 409 | 25 mg | 11005 |
| Amplite™ Red | Fluorogenic | 571 | 584 | 1000 Assays | 11011 |
| Amplite™ IR | Fluorogenic | 648 | 668 | 1 mg | 11009 |
| Luminol | Chemiluminescent | 355 | 412 | 1 g | 11050 |
Table 9. AP-Streptavidin Conjugate & Substrates
| Product | Detection | Abs/Ex (nm) | Ex (nm) | Unit Size | Cat No. |
| pNPP *CAS 4264-83-9* | Chromogenic | 405 | 25 mg | 11619 | |
| FDP *CAS 217305-49-2* | Fluorogenic | 498 | 517 | 5 mg | 11600 |
| MUP, disodium salt *CAS 22919-26-2* | Fluorogenic | 360 | 448 | 25 mg | 11610 |
| MUP, disodium salt *CAS 22919-26-2* | Fluorogenic | 360 | 448 | 10 g | 11612 |
| DiFMUP | Fluorogenic | 358 | 450 | 5 mg | 11627 |
| SunRed™ Phosphate | Fluorogenic | 653 | 661 | 5 mg | 11629 |
| D-Luciferin phosphate *CAS 145643-12-3 | Chemiluminescent | 5 mg | 12512 |
Fluorescent Streptavidin Conjugates
Fluorescent streptavidin conjugates are widely used in fluorescence imaging to detect biotinylated antibodies, ligands and DNA probes for in situ hybridization techniques, immunohistochemistry and multicolor flow cytometry. We offer a comprehensive portfolio of fluorescent streptavidin labeled with iFluor™ dyes, mFluor™ dyes, phycobiliproteins and tandem dyes, and more. Benefits of our iFluor™ streptavidin conjugates include exceptionally bright fluorescence, excellent photostability and pH insensitive fluroescence over a wide molar range.
iFluor™ Streptavidin Conjugates
Streptavidin conjugates labeled with iFluor™ dyes exhibit exceptionally bright and photostable fluorescence, outperforming Alexa Fluor® and other spectrally similar conjugtes. iFluor™ streptavidin conjugates are designed for use in a variety of cell analysis and protein analysis applications including imaging, flow cytometry and fluorescent western blotting. As a precaution, avoid using blue fluorescent streptavidin conjugates when detecting low abundance targets. Blue fluorescent dyes have lower fluorescence and can experience higher non-specific background than other dyes.
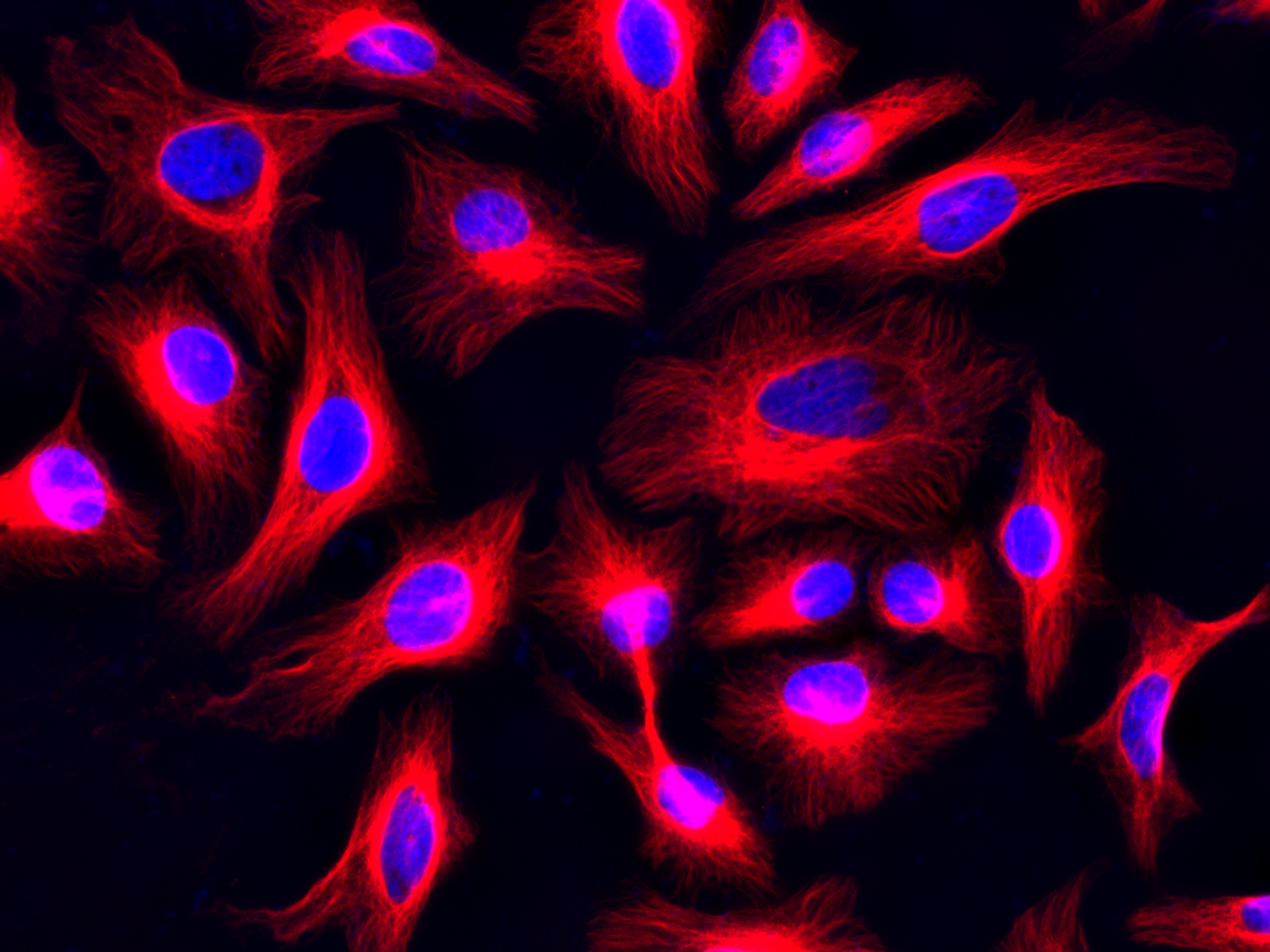
Figure 5. Immunofluorescent stain of &alpha-tubulin in HeLa cells. &alpha-tubulin in fixed and permeabilized HeLa cells were incubated with rabbit anti-tubulin primary antibody, followed by incubation with biotinylated goat anti-rabbit IgG (H&L) (Cat No. 16794), and then visualized with iFluor™ 647-streptavidin conjugate (Cat No. 16966. Nuclei were counterstained using DAPI (Cat No. 17507.)
Table 10. iFluor-Streptavidin Conjugates For Imaging and Flow Cytometry
| Product | Ex/Em (nm) | Filter Set | Ext. Coeff.¹ | FQY² | Unit Size | Cat No. |
| iFluor™ 350 Streptavidin | 344/448 | DAPI | 20,000 | 0.95 | 1 mg | 16980 |
| iFluor™ 405 Streptavidin | 402/425 | DAPI | 37,000 | 0.91 | 1 mg | 16982 |
| iFluor™ 488 Streptavidin | 491/516 | FITC | 75,000 | 0.9 | 1 mg | 16985 |
| iFluor™ 514 Streptavidin | 527/554 | TRITC | 80,000 | 0.83 | 1 mg | 16986 |
| iFluor™ 532 Streptavidin | 543/563 | TRITC | 90,000 | 0.68 | 1 mg | 16987 |
| iFluor™ 546 Streptavidin | 541/557 | TRITC | 100,000 | 0.67 | 200 µg | 16958 |
| iFluor™ 555 Streptavidin | 556/569 | TRITC | 100,000 | 0.64 | 1 mg | 16989 |
| iFluor™ 568 Streptavidin | 568/587 | TRITC | 100,000 | 0.57 | 200 µg | 16960 |
| iFluor™ 594 Streptavidin | 587/603 | Texas Red | 180,000 | 0.53 | 1 mg | 16992 |
| iFluor™ 633 Streptavidin | 638/652 | Texas Red | 250,000 | 0.29 | 1 mg | 16995 |
| iFluor™ 647 Streptavidin | 654/669 | Cy5 | 250,000 | 0.25 | 1 mg | 16996 |
| iFluor™ 680 Streptavidin | 683/700 | Cy5.5 | 220,000 | 0.23 | 1 mg | 16997 |
| iFluor™ 700 Streptavidin | 690/713 | Cy5.5 | 220,000 | 0.23 | 1 mg | 16998 |
| iFluor™ 750 Streptavidin | 756/777 | Cy7 | 275,000 | 12 | 1 mg | 16999 |
| iFluor™ 790 Streptavidin | 786/811 | Cy7 | 250,000 | 0.13 | 200 µg | 16975 |
| iFluor™ 800 Streptavidin | 801/820 | Cy7 | 250,000 | 0.11 | 200 µg | 16976 |
| iFluor™ 820 Streptavidin | 820/849 | Cy7 | 250,000 | N/D³ | 200 µg | 16977 |
| iFluor™ 840 Streptavidin | 836/876 | Cy7 | 200,000 | N/D³ | 200 µg | 16978 |
| iFluor™ 860 Streptavidin | 852/877 | Cy7 | 250,000 | N/D³ | 200 µg | 16979 |
Note
- Ext. Coeff. = Extinction coefficient at their maximum absorption wavelength. The units of extinction coefficient are cm-1M-1.
- FQY = fluorescence quantum yield in aqueous buffer (pH 7.2).
- N/D = Not determined.
mFluor™ Streptavidin Conjugates for Flow Cytometry
Streptavidin conjugates labeled with mFluor™ dyes exhibit minimal self-quenching, producing intensely fluorescent and photostable conjugates for flow cytometery. mFluor™ streptavidin conjugates are designed for optimal excitation by one of the major laser lines commonly equipped in flow cytometers, such as the 405 nm, 488 nm, 532 nm, 561 nm or 633 nm laser lines.
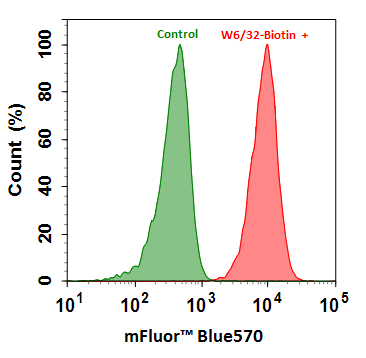
Figure 6. Flow cytometry analysis of HL-60 cells stained with (Red) or without (Green) 1ug/ml Anti-Human HLA-ABC-Biotin and then followed by mFluor™ Blue 570-streptavidin conjugate (Cat No.16935).
Table 11. mFluor™ Streptavidin Conjugates for Flow Cytometry
| Streptavidin Conjugates | Ex (nm) | Em (nm) | Filter Set | Cat No. |
| mFluor™ Violet 450 Streptavidin | 405 | 444 | 450/40 | 16930 |
| mFluor™ Violet 510 Streptavidin | 408 | 503 | 525/50 | 16931 |
| mFluor™ Violet 540 Streptavidin | 393 | 536 | 525/50 | 16932 |
| mFluor™ Blue 570 Streptavidin | 505 | 564 | 575/26 | 16935 |
| mFluor™ Green 620 Streptavidin | 522 | 621 | 610/20 | 16938 |
| mFluor™ Yellow 630 Streptavidin | 609 | 626 | 610/20 | 16942 |
| mFluor™ Red 700 Streptavidin | 685 | 704 | 730/45 | 16946 |
| mFluor™ Red 780 Streptavidin | 629 | 780 | 780/60 | 16948 |
Streptavidin APC, PE, PerCP and Tandem Dye Conjugates for Flow Cytometry
Streptavidin PE, APC and PerCP conjugates are commonly used in applications that require high sensitivity, but not phostostability, primarily flow cytometry, FACS, immunophenotyping, and microarrays. The unusually bright fluorescence of phycobiliproteins is due to their unusually high extinction coefficients and quantum yields.
Tandem dye conjugates compromise of a donor phycobiliprotein (e.g. APC or PE) labeled to acceptor iFluor™ dyes that emit fluorescence at a longer-wavelength. Using fluorescence resonance energy transer (FRET), light absorbed by the the donor phycobiliprotein results in fluorescence emission of the acceptor. In flow cytometry, tandem dyes are ideally suited for multicolor parametric analysis of cells due to their exploitation of a single excitation source and their significantly large Stokes shifts.
Table 12. Streptavidin APC, PE, PerCP and Tandem Dye Conjugates for Flow Cytometry
| Streptavidin Conjugates | Ex (nm) | Em (nm) | Filter Set | Cat No. |
| RPE Streptavidin | 564 | 574 | 575/26 | 16900 |
| APC Streptavidin | 651 | 660 | 660/20 | 16902 |
| PerCP Streptavidin | 477 | 678 | 695/40 | 16905 |
| RPE-iFluor™ 647 Streptavidin | 568 | 666 | 670/14 | 16906 |
| RPE-iFluor™ 750 Streptavidin | 566 | 778 | 780/60 | 16907 |
| APC-iFluor™ 750 Streptavidin | 651 | 791 | 780/60 | 16908 |
| RPE-Cy7 Streptavidin | 566 | 778 | 780/60 | 16916 |
Streptavidin-Xtra™ Conjugates
The optimal degree of labeling of Streptavidin-Xtra™ iFluor™ conjugates produce the brightest fluorescent streptavidin conjugates for the detection of biotinylated antibodies in cell imaging, flow cytometry and western blot applications. Streptavidin-Xtra™ iFluor™ conjugates image low-abundance targets with greater sensitivity and detail compared to traditional streptavidin Alexa Fluor® conjugates.
Advantages
- Bright and photostable fluorescence without significant self-quenching
- High siganl-to-noise ratios: 3∼5 fold signal improvement versus Alexa Fluor®
- pH insensitive fluroescence over a wide molar range
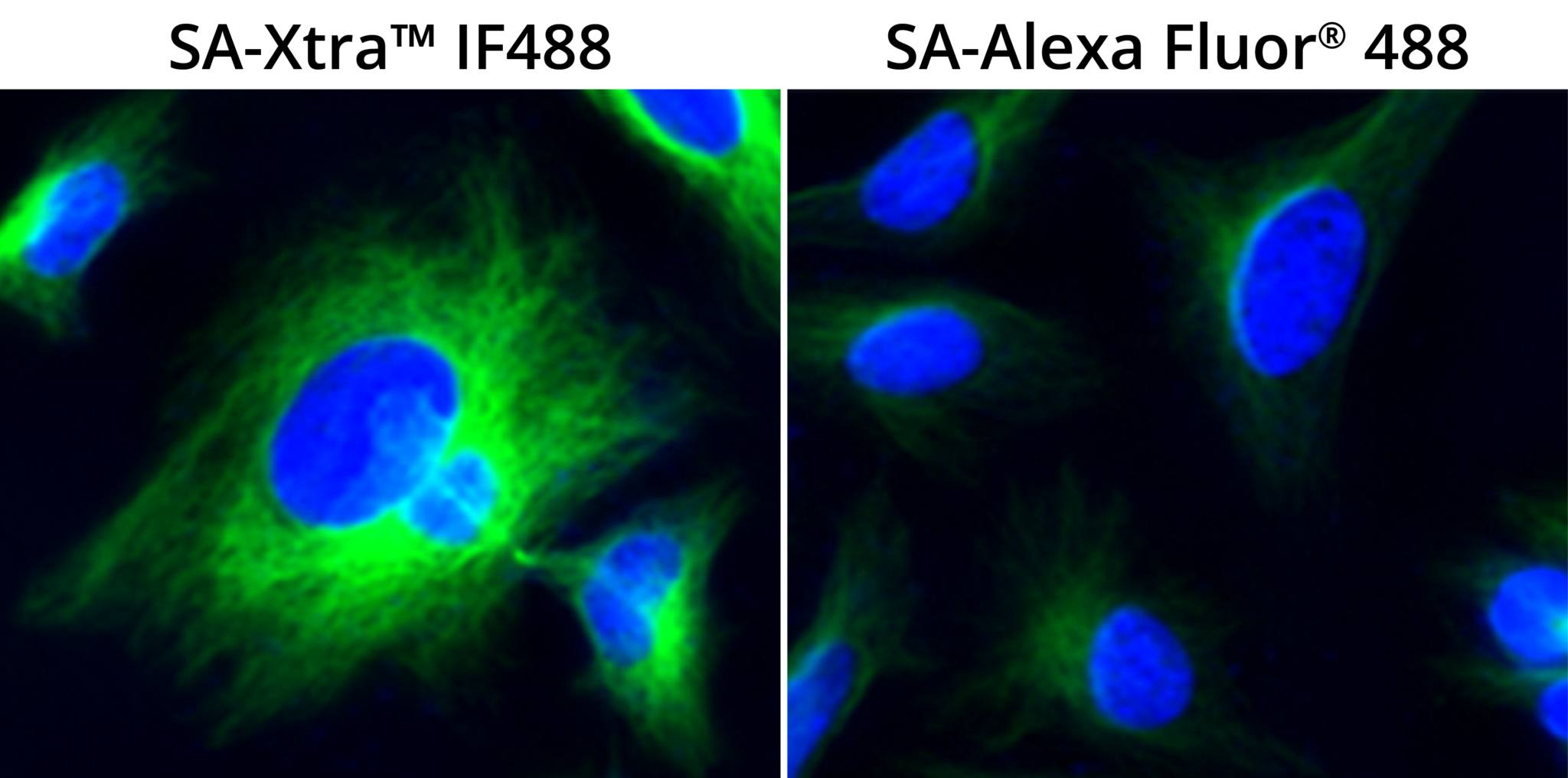
Figure 7. Comparison of HeLa cells stained with Streptavidin-Xtra™ IF488 and Streptavidin-Alexa Fluor® 488.HeLa cells were fixed with 4% paraformaldehyde for 30 minutes, permeabilized with 0.02% Triton™ X-100 for 10 minutes, blocked with 1% BSA for 1 hour and labeled with 1 µg/mL α-tubulin Mouse mAb for 1 hour at room temperature. Biotinylated goat anti-mouse IgG (H+L) (Cat No. 16729) was used for detection α-tubulin and visualized with Streptavidin-Xtra™ iFluor 488 (Cat No. 46001) and Streptavidin-Alexa Fluor® 488. Cells were counterstained with Hoechst 33342 (Cat No. 17535).
Table 13. Streptavidin-Xtra™ Conjugates
| Streptavidin Conjugates | Ex (nm) | Em (nm) | Filter Set | Cat No. |
| Streptavidin-Xtra™ iFluor™ 488 | 491 | 516 | FITC | 46001 |
| Streptavidin-Xtra™ iFluor™ 555 | 556 | 569 | TRITC | 46003 |
| Streptavidin-Xtra™ iFluor™ 594 | 587 | 603 | Texas Red | 46005 |
| Streptavidin-Xtra™ iFluor™ 647 | 654 | 669 | Cy5 | 46007 |
Product Ordering Information
Table 14. Ordering Info for Biotin Products
| Cat# | Product Name | Unit Size | Ex | Em |
| 3001 | Biotin *CAS 58-85-5* | 1 g | ||
| 3002 | Biotin, succinimidyl ester *CAS 35013-72-0* | 100 mg | ||
| 3003 | Biotin ethylenediamine *CAS 216299-38-6* | 10 mg | ||
| 3004 | Biotin cadaverine | 25 mg | ||
| 3005 | Biotin C2 maleimide | 25 mg | ||
| 3006 | Biotin-4-fluorescein *CAS 1032732-74-3* | 5 mg | 492 | 518 |
| 3007 | Biotin hydrazide *CAS 66640-86-6* | 25 mg | ||
| 3009 | Biotin-X NTA [Biotin-X nitrilotriacetic acid, potassium salt] *CAS 856661-92-2* | 1 mg | ||
| 3010 | Biotin-X, succinimidyl ester *CAS 72040-63-2* | 25 mg | ||
| 3014 | Biotin PEG2 amine *CAS 138529-46-1* | 5 mg | ||
| 3015 | Biotin PEG2 maleimide *CAS 305372-39-8* | 5 mg | ||
| 3016 | Biotin PEG2 succinimidyl ester | 25 mg | ||
| 3017 | Fluorescein biotin | 5 mg | 492 | 515 |
| 3019 | Biotin-PEG3-azide *CAS 945633-30-7* | 5 mg | ||
| 3020 | Biotin Azide | 5 mg | ||
| 3021 | Biotin Alkyne *CAS 1006592-45-5* | 5 mg | ||
| 3022 | Biotin PEG4 succinimidyl ester | 25 mg | ||
| 3050 | ReadiView™ biotin acid | 25 mg | ||
| 3053 | ReadiView™ biotin amine | 5 mg | ||
| 3055 | ReadiView™ biotin hydrazide | 5 mg | ||
| 3058 | ReadiView™ biotin maleimide | 5 mg | ||
| 3059 | ReadiView™ biotin succinimidyl ester | 5 mg | ||
| 5520 | ReadiLink™ Protein Biotinylation Kit | 2 Labelings | ||
| 5521 | ReadiLink™ Protein Biotinylation Kit *Powered by ReadiView™ Biotin Visionization Technology* | 1 kit | ||
| 5522 | Amplite™ Colorimetric Biotin Quantitation Kit | 200 Tests | 500 | |
| 16729 | Biotinylated goat anti-mouse IgG (H+L) | 1 mg | ||
| 16794 | Biotinylated goat anti-rabbit IgG (H+L) | 1 mg | ||
| 17016 | Biotin-11-dUTP *1 mM in Tris Buffer (pH 7.5)* *CAS 86303-25-5* | 25 nmoles | ||
| 17017 | Biotin-16-dUTP *1 mM in Tris Buffer (pH 7.5)* *CAS 136632-31-0* | 25 nmoles | ||
| 17018 | Biotin-20-dUTP *1 mM in Tris Buffer (pH 7.5)* | 25 nmoles | ||
| 20605 | Cal-520®-Biotin Conjugate | 5×50 ug | 492 | 514 |
Table 15. Ordering Info for Streptavidin Products
| Cat# | Product Name | Unit Size |
| 16900 | RPE-streptavidin conjugate | 100 ug |
| 16901 | RPE-streptavidin conjugate | 1 mg |
| 16902 | APC-streptavidin conjugate | 100 ug |
| 16905 | PerCP-streptavidin conjugate | 100 ug |
| 16906 | RPE-iFluor™ 647-streptavidin conjugate | 100 ug |
| 16907 | RPE-iFluor™ 750-streptavidin conjugate | 100 ug |
| 16908 | APC-iFluor™ 750-streptavidin conjugate | 100 ug |
| 16910 | FITC-streptavidin conjugate | 1 mg |
| 16911 | Texas Red®-streptavidin conjugate | 1 mg |
| 16912 | Cy3®-streptavidin conjugate | 1 mg |
| 16913 | Cy5®-streptavidin conjugate | 1 mg |
| 16914 | Cy7®-streptavidin conjugate | 1 mg |
| 16920 | HRP-streptavidin conjugate | 1 mg |
| 16921 | AP-streptavidin conjugate [Streptavidin-alkaline phosphatase conjugate] | 1 mg |
| 16925 | trFluor™ Eu-streptavidin conjugate | 100 ug |
| 16926 | trFluor™ Tb-streptavidin conjugate | 100 ug |
| 16930 | mFluor™ Violet 450-streptavidin conjugate | 100 ug |
| 16931 | mFluor™ Violet 510-streptavidin conjugate | 100 ug |
| 16932 | mFluor™ Violet 540-streptavidin conjugate | 100 ug |
| 16935 | mFluor™ Blue 570-streptavidin conjugate | 100 ug |
| 16938 | mFluor™ Green 620-streptavidin conjugate | 100 ug |
| 16942 | mFluor™ Yellow 630-streptavidin conjugate | 100 ug |
| 16946 | mFluor™ Red 700-streptavidin conjugate | 100 ug |
| 16948 | mFluor™ Red 780-streptavidin conjugate | 100 ug |
| 16950 | iFluor™ 350-streptavidin conjugate | 200 ug |
| 16952 | iFluor™ 405-streptavidin conjugate | 200 ug |
| 16955 | iFluor™ 488-streptavidin conjugate | 200 ug |
| 16956 | iFluor™ 514-streptavidin conjugate | 200 ug |
| 16957 | iFluor™ 532-streptavidin conjugate | 200 ug |
| 16958 | iFluor™ 546-streptavidin conjugate | 200 ug |
| 16959 | iFluor™ 555-streptavidin conjugate | 200 ug |
| 16960 | iFluor™ 568-streptavidin conjugate | 200 ug |
| 16962 | iFluor™ 594-streptavidin conjugate | 200 ug |
| 16965 | iFluor™ 633-streptavidin conjugate | 200 ug |
| 16966 | iFluor™ 647-streptavidin conjugate | 200 ug |
| 16968 | iFluor™ 680-streptavidin conjugate | 200 ug |
| 16970 | iFluor™ 700-streptavidin conjugate | 200 ug |
| 16973 | iFluor™ 750-streptavidin conjugate | 200 ug |
| 16980 | iFluor™ 350-streptavidin conjugate | 1 mg |
| 16982 | iFluor™ 405-streptavidin conjugate | 1 mg |
| 16985 | iFluor™ 488-streptavidin conjugate | 1 mg |
| 16986 | iFluor™ 514-streptavidin conjugate | 1 mg |
| 16987 | iFluor™ 532-streptavidin conjugate | 1 mg |
| 16989 | iFluor™ 555-streptavidin conjugate | 1 mg |
| 16992 | iFluor™ 594-streptavidin conjugate | 1 mg |
| 16995 | iFluor™ 633-streptavidin conjugate | 1 mg |
| 16996 | iFluor™ 647-streptavidin conjugate | 1 mg |
| 16997 | iFluor™ 680-streptavidin conjugate | 1 mg |
| 16998 | iFluor™ 700-streptavidin conjugate | 1 mg |
| 16999 | iFluor™ 750-streptavidin conjugate | 1 mg |