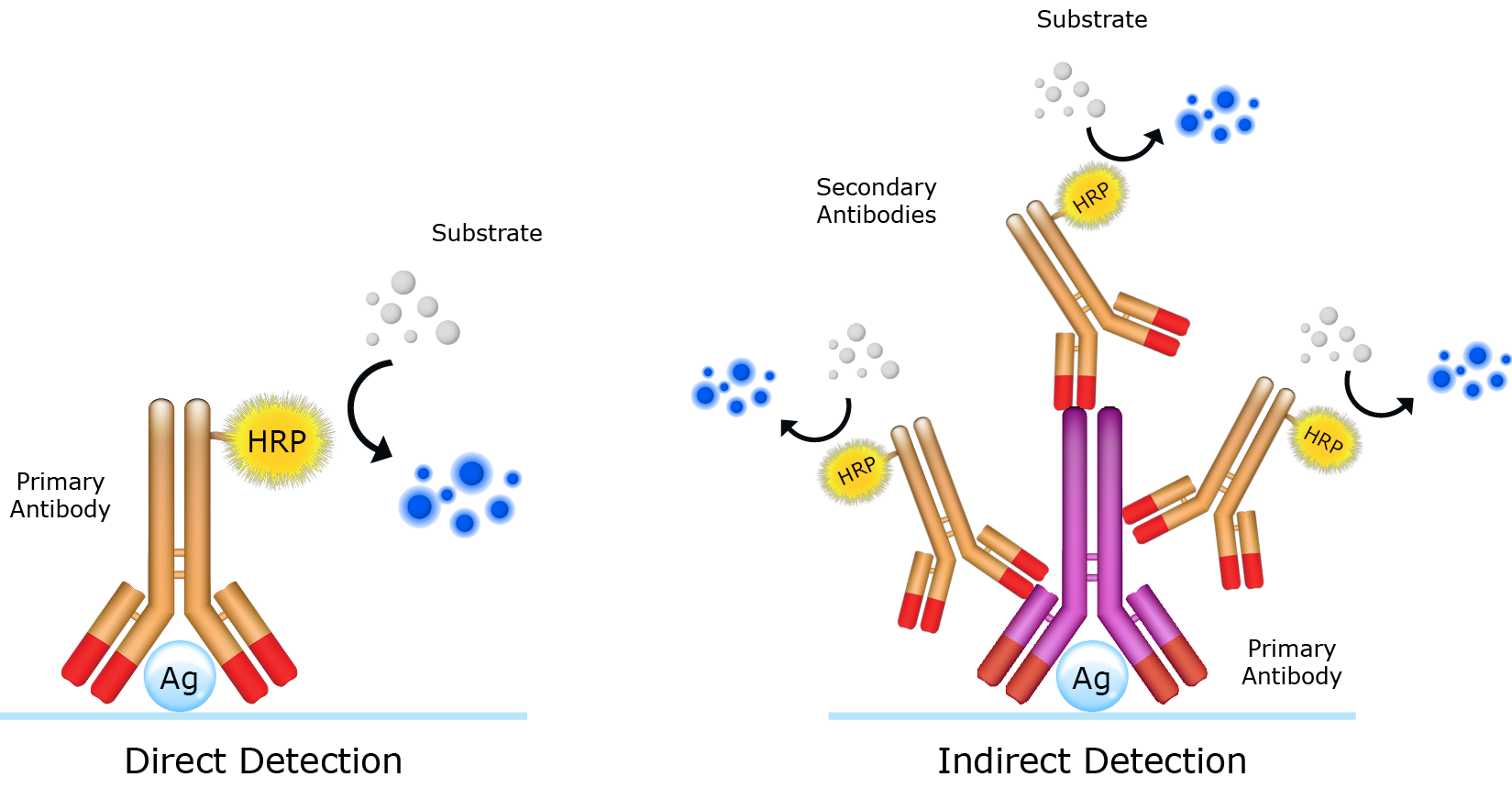
Using secondary antibodies
Secondary antibodies are key components of the indirect detection method. Used in sequence with primary antibodies, secondary antibodies facilitate in the detection, quantification and purification of target antigens by binding to the primary antibody, which directly binds to the antigen. Reporter molecules (e.g. an enzyme or a fluorophore) conjugated to the secondary antibodies enables visualization of the immune complex. The choice of reporter molecule is contingent upon the application and how the secondary antibody will be detected. While the use of secondary antibodies requires an additional immunolabeling step, it presents numerous advantages over the direct detecion method, namely signal amplification and flexibility. Signal amplification arises from the specificity of the secondary antibody for a designated reigion on the primary antibody (e.g. Fc region). This permits multiple secondary antibodies to bind to a single primary antibody, amplifying the signal and improving sensitivity (Figure 1). The use of secondary antibodies also affords the user flexibility contributed by the diversity of available reporter molecules. For example, iFluor™ 800 dye-labeled and iFluor™ 680 dye-labeled secondary antibodies enable two-color imaging in protein detection applications such Western blot.
Select the correct reporter molecule for your application
Secondary antibodies are generally supplied conjugated to a detectable label such as an enzyme, fluorophore, or biotin. The choice of label depends upon the downstream application for which the secondary antibody will be used and detected.
Table 1. Common Applications for Secondary Antibody Conjugates
Enzyme-labeled secondary antibodies and other reagents for ELISA
Monoclonal antibodies, polyclonal antibodies or a combination of both are typically used in ELISA assays as either detection or capture antibodies. Monoclonal antibodies are inherently monovalent, with specificity towards a single epitope per antigen. In all ELISA formats, monoclonal antibodies are commonly used as detection antibodies. Because they seldom cross-react with other proteins, they are less likely to generate non-specific signals. In contrast, polyclonal antibodies, which are a complex mixture of antibodies, recognize multiple epitopes found in a single antigen. While they are often used in sandwich ELISA assays as ‘capture antibodies’ to pull down as much of the antigen as possible, they can also be used as detection antibodies. Polyclonal antibodies are highly susceptible to batch-to-batch variation, and should be thoroughly tested and validated prior to using.
HRP and poly-HRP secondary antibodies for ELISA
The enzyme horseradish perxoidase ((HRP, Cat No. 11025) is commonly conjugated to secondary antibodies, and used in either indirect or sandwich ELISA assays. The widespread adoption of HRP as a reporter in ELISA is primarly due to three factors. First, HRP has the capacity to amplify weak signal and enhance the detectability of poorly-expressed antigens. Second, the relatively small size of HRP (?44 kDa) compared to other reporter enzymes (e.g. alkaline phosphatase (~140 kDa)) further improves intracellular penetration into samples, reduces steric hindrance and minimizes immunoreactivity loss. Third, is the high turnover rate and good stability of HRP that enables rapid and strong signal generation. AAT Bioquest offers highly purified and cross-adsorbed secondary antibodies conjugated to HRP and poly-HRP. MegaWox™ poly-HRP secondary conjugates are designed to deliver high levels of sensitivity and low background in ELISA assays. Use MegaWox™ poly-HRP conjugates in immunoassays where sample volume is limited or when the target molecule is poorly-expressed.
Table 2. Available HRP-Secondary Antibody Conjugates for ELISA
| Cat# |
Product Name |
Unit Size |
| 11035 |
MegaWox™ polyHRP-Goat Anti-Mouse IgG Conjugate |
1 mg |
| 11037 |
MegaWox™ polyHRP-Goat Anti-Rabbit IgG Conjugate |
1 mg |
| 16728 |
HRP Goat Anti-mouse IgG (H+L) Antibody |
1 mg |
| 16793 |
HRP Goat Anti-rabbit IgG (H+L) Antibody |
1 mg |
| 50261 |
HRP Goat Anti-human IgG (H+L) Antibody |
1 mg |
Fluorimetric substrates
Fluorimetric (or fluorogenic) substrates react with enzymes to generate highly fluorescent byproducts that can be measured using a fluorescence plate reader. The degree of photon emission following light excitation is proportional to the amount of antigen in the sample. AAT Bioquest provides fluorimetric substrates for horseradish peroxidase (HRP) and alkaline phosphatase (AP) ranging in sensitivity and fluorescence properties.
Table 4. Available Fluorimetric Enzyme Substrates for ELISA
| Enzyme |
Substrate |
Ex (nm) |
Em (nm) |
Unit Size |
Cat No. |
| AP |
MUP, disodium salt |
360 nm |
448 nm |
25 mg |
11610 |
| AP |
MUP, disodium salt |
360 nm |
448 nm |
10 g |
11612 |
| AP |
MUP, free acid |
360 nm |
448 nm |
25 mg |
11614 |
| AP |
MUP, free acid |
360 nm |
448 nm |
5 g |
11617 |
| AP |
DiFMUP |
360 nm |
450 nm |
5 mg |
11627 |
| AP |
FDP |
497 nm |
516 nm |
5 mg |
11600 |
| AP |
PhosLite™ Green |
345 nm |
520 nm |
1 mg |
11630 |
| AP |
SunRed™ Phosphate |
652 nm |
660 nm |
5 mg |
11629 |
| HRP |
Amplite™ Blue |
324 nm |
409 nm |
25 mg |
11005 |
| HRP |
Amplite™ ADHP |
570 nm |
583 nm |
25 mg |
11000 |
| HRP |
Amplite™ Red |
570 nm |
583 nm |
1000 Assays |
11011 |
| HRP |
Amplite™ IR |
646 nm |
667 nm |
1 mg |
11009 |
Chemiluminescent substrates
Chemiluminescent substrates react with enzymes to generate luminescent byproducts that can be measured using a luminometer. Since the emission of a photon is a result of a chemical reaction and not light excitation, there is minimal background interference. AAT Bioquest provides chemiluminescent substrates for horseradish peroxidase (HRP) and alkaline phosphatase (AP) ranging in sensitivity and chemiluminescence properties.
Table 5. Chemiluminescent substrates For Substrates and detection strategies for enzyme-labeled secondary antibodies
| Enzyme |
Substrate |
Em (nm) |
Unit Size |
Cat No. |
| AP |
D-Luciferin phosphate |
|
1 mg |
12512 |
| HRP |
Luminol |
410 nm |
1 mg |
11050 |