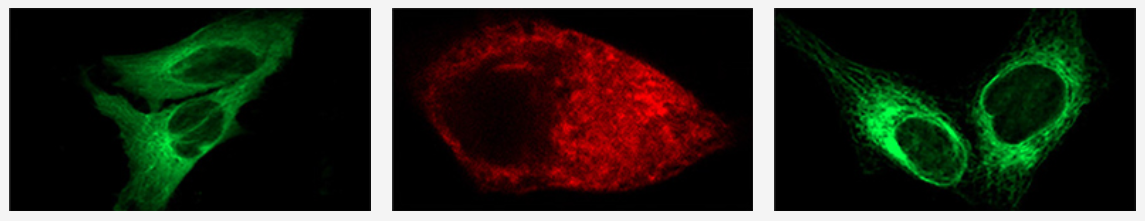
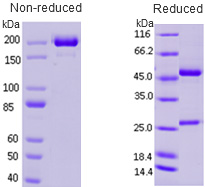
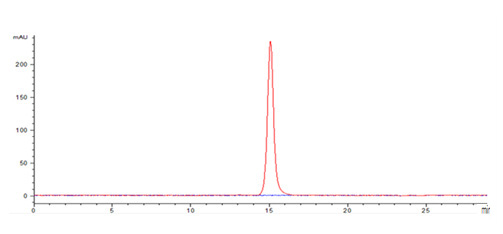
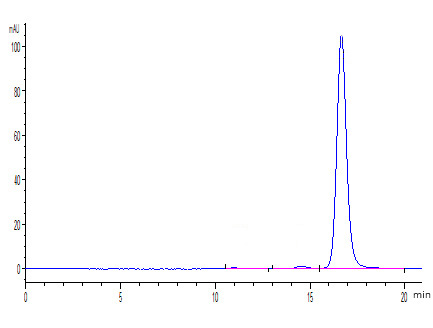
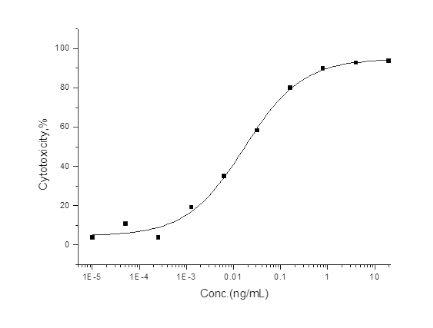
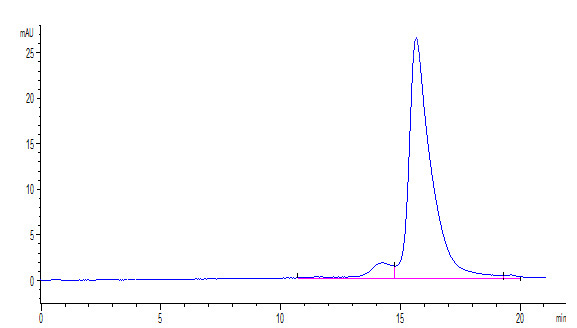
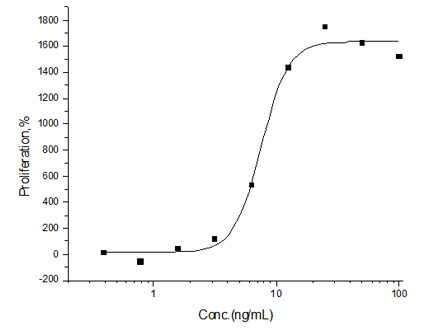
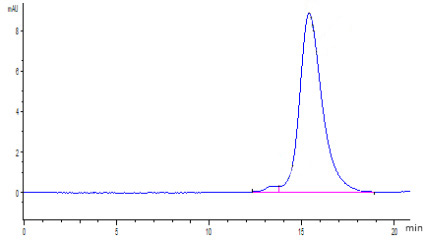
Cancer biomarkers can be used for prognosis: to predict the natural course of a tumor, indicating whether the outcome for the patient is likely to be good or poor (prognosis). They can also help doctors to decide which patients are likely to respond to a given drug (prediction) and at what dose it might be most effective (pharmacodynamics). Cancer biomarkers are present in tumor tissues or serum and encompass a wide variety of molecules, including DNA, mRNA, transcription factors, cell surface receptors, and secreted proteins. One of these serum biomarkers in wide use is PSA which is produced by normal prostate cells. The higher the PSA is in the serum, the higher the correlation is toward the existence of prostate cancer.
Prognostic biomarkers allow the natural course of a tumor to be predicted, distinguishing ‘good outcome’ tumors from ‘poor outcome’ tumors, and they guide the decision of whom to treat (or how aggressively to treat). Predictive biomarkers are used to assess the probability that a patient will benefit from a particular treatment. For example, patients with breast cancer in which the gene encoding the oestrogen receptor is expressed respond to treatment with tamoxifen, whereas when the gene ERBB2 (also known as HER2) is amplified in the tumour, the patients benefit from treatment with trastuzumab (Herceptin) instead. Pharmacodynamic biomarkers measure the near-term treatment effects of a drug on the tumor (or on the host) and can, in theory, be used to guide dose selection in the early stages of clinical development of a new anticancer drug.
The application of cancer biomarkers is still controversial. PSA is a widely used cancer biomarker. However, there are reasons other than cancer that can cause rises in PSA, such as infections within the prostate gland, increased exercise with irritation of the affected area, and even vigorous physical examination by a doctor. Cancer antigen 125 (CA-125) can be a biomarker of ovarian cancer risk or an indicator of malignancy, but it has low sensitivity and specificity. Levels of this marker can be high in people who have pancreatitis, kidney or liver disease, making its accuracy as a cancer diagnostic tool very limited. Carcinoembryonic antigen (CEA) is another biomarker that is elevated in patients with colorectal, breast, lung, or pancreatic cancer. As a screening test, it can be elevated by many other factors than cancer; smoking for instance raises CEA levels.
An ideal tumor marker should be measured easily, reliably and cost-effectively using an assay with high analytical sensitivity and specificity. In addition, an ideal tumor marker should be present in detectable quantities at early or preclinical stages and the quantitative levels of the tumor marker should reflect tumor burden. Recent technological advances, especially in the fields of genomics and proteomics, have made it easier to identify many biomarkers at once in high-throughput screens. The validation of cancer biomarkers – that is, determination of clinical relevance and applicability – is also quite challenging, and many questions have been raised regarding how new tests will be developed, evaluated, and integrated into clinical practice.